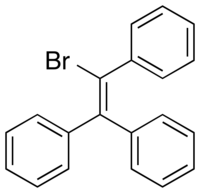
Triphenylbromoethylene
Appearance
 | |
| Clinical data | |
|---|---|
| Trade names | Bromylene, Eitriphin, Oestronyl, Prostilban, Tribenorm |
| Other names | TPBE; Tribromophenylethylene; Bromotriphenylethylene; Phenylstilbene bromide; Fenbrostilbenum |
| Drug class | Nonsteroidal estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.015.029 |
| Chemical and physical data | |
| Formula | C20H15Br |
| Molar mass | 335.244 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Triphenylbromoethylene (TPBE; brand names Bromylene, Eitriphin, Oestronyl, Prostilban, Tribenorm), also known as bromotriphenylethylene or as phenylstilbene bromide, is a synthetic nonsteroidal estrogen of the triphenylethylene group that was marketed in the 1940s similarly to the closely related estrogen triphenylchloroethylene.[1][2]
A diethoxylated derivative of triphenylbromoethylene, estrobin (DBE), is also an estrogen, but, in contrast, was never marketed.[3] An ethylated derivative of triphenylbromoethylene, broparestrol (BDPE), is a selective estrogen receptor modulator (SERM) that has been marketed.[4][5]
See also
References
- ^ Negwer M, Scharnow HG (2001). Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. p. 1861. ISBN 978-3-527-30247-5.
C20H15Br. 1607-57-4. Bromotriphenylethene = Triphenylbromoethylene = Phenylstilbene bromide = 1,1',1"-(1-Bromo-1-ethenyl-2-yli- dene)tris[benzene] (•) S Bromylene, Fenbrostilbenum, Oestronyl, Phenylstilbene bromide, Prostilban, Tribenorm U Synthetic estrogen
- ^ Paterson E, Gilbert CW (May 1949). "Metabolism of the oestrogen triphenylbromoethylene". Nature. 163 (4151): 801–802. Bibcode:1949Natur.163..801P. doi:10.1038/163801a0. PMID 18128458. S2CID 13052481.
- ^ Emmens CW (22 October 2013). Hormone Assay. Elsevier Science. pp. 394–. ISBN 978-1-4832-7286-3.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 183–. ISBN 978-1-4757-2085-3.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 139–. ISBN 978-3-88763-075-1.
