Trimethoprim
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /traɪˈmɛθəprɪm/ |
| Trade names | Proloprim, Monotrim, Triprim, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684025 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Diaminopyrimidines |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90–100% |
| Protein binding | 44% |
| Metabolism | Liver |
| Elimination half-life | 8–12 hours |
| Excretion | Kidney (50–60%), faeces (4%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.915 |
| Chemical and physical data | |
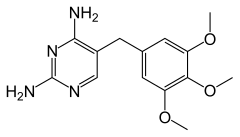
| Formula | C14H18N4O3 |
| Molar mass | 290.323 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Trimethoprim (TMP) is an antibiotic used mainly in the treatment of bladder infections.[1] Other uses include for middle ear infections and travelers' diarrhea.[1] With sulfamethoxazole or dapsone it may be used for Pneumocystis pneumonia in people with HIV/AIDS.[1][2] It is taken orally (swallowed by mouth).[1]
Common side effects include nausea, changes in taste, and rash.[1] Rarely it may result in blood problems such as not enough platelets or white blood cells.[1] Trimethoprim may cause sun sensitivity.[1] There is evidence of potential harm during pregnancy in some animals but not humans.[3] It works by blocking folate metabolism via dihydrofolate reductase in some bacteria, preventing creation of bacterial DNA and RNA and leading to bacterial cell death.[1]
Trimethoprim was first used in 1962.[4] It is on the World Health Organization's List of Essential Medicines.[5] It is available as a generic medication.[6]
Medical uses
It is primarily used in the treatment of urinary tract infections, although it may be used against any susceptible aerobic bacterial species.[7] It may also be used to treat and prevent Pneumocystis jirovecii pneumonia.[7] It is generally not recommended for the treatment of anaerobic infections such as Clostridioides difficile colitis (the leading cause of antibiotic-induced diarrhea).[7] Trimethoprim has been used in trials to treat retinitis.[8]
Resistance to trimethoprim is increasing, but it is still a first-line antibiotic in many countries.[9]
Spectrum of susceptibility
Cultures and susceptibility tests should be done to make sure bacteria are treated by trimethoprim.[10][11]
- Escherichia coli
- Proteus mirabilis
- Klebsiella pneumoniae
- Enterobacter species
- Coagulase-negative Staphylococcus species, including S. saprophyticus
- Streptococcus pneumoniae
- Haemophilus influenzae
Side effects
Common
Rare
- Can cause thrombocytopenia (low levels of platelets) by lowering folic acid levels; this may also cause megaloblastic anemia.[14]
- Trimethoprim antagonizes the epithelial sodium channel in the distal tubule, thus acting like amiloride. This can cause increased potassium levels in the body (hyperkalemia).[15]
- Can compete with creatinine for secretion into the renal tubule. This can cause an artificial rise in the serum creatinine.[16]
- Use in EHEC infections may lead to an increase in expression of Shiga toxin.[17]
Contraindications
- Known hypersensitivity to trimethoprim
- History of megaloblastic anemia due to folate deficiency[18]
It may be involved in a reaction similar to disulfiram when alcohol is consumed after it is used, in particular when used in combination with sulfamethoxazole.[19][20]
Pregnancy
Based on the studies that show that trimethoprim crosses the placenta and can affect folate metabolism, there has been growing evidence of the risk of structural birth defects associated with trimethoprim, especially during the first trimester of pregnancy.[21]
The trophoblasts in the early fetus are sensitive to changes in the folate cycle. A 2013 study found a doubling in the risk of miscarriage in women exposed to trimethoprim in the early pregnancy.[22]
Mechanism of action

Trimethoprim binds to dihydrofolate reductase and inhibits the reduction of dihydrofolic acid (DHF) to tetrahydrofolic acid (THF).[24] THF is an essential precursor in the thymidine synthesis pathway and interference with this pathway inhibits bacterial DNA synthesis.[24] Trimethoprim's inhibitory activity for bacterial dihydrofolate reductase is sixty thousand times greater than for human dihydrofolate reductase.[25] Sulfamethoxazole inhibits dihydropteroate synthase, an enzyme involved further upstream in the same pathway.[24] Trimethoprim and sulfamethoxazole are commonly used in combination due to possible synergistic effects, and reduced development of resistance.[24] This benefit has been questioned.[26]

History
Trimethoprim was first used in 1962.[4] In 1972, it was used as a prophylactic treatment for urinary tract infections in Finland.[4]
Its name is derived from trimethyloxy-pyrimidine.
References
- ^ a b c d e f g h "Trimethoprim". The American Society of Health-System Pharmacists. Archived from the original on 24 September 2015. Retrieved 1 August 2015.
- ^ Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE (May 2014). "Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated Guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America". Clinical Infectious Diseases. 58 (9): 1308–1311. doi:10.1093/cid/ciu094. PMC 3982842. PMID 24585567.
- ^ "Prescribing medicines in pregnancy database". Australian Government. 3 March 2014. Archived from the original on 8 April 2014. Retrieved 22 April 2014.
- ^ a b c Huovinen P (June 2001). "Resistance to trimethoprim-sulfamethoxazole". Clinical Infectious Diseases. 32 (11): 1608–1614. doi:10.1086/320532. PMID 11340533.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 113. ISBN 978-1-284-05756-0.
- ^ a b c Rossi S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ^ Pradhan E, Bhandari S, Gilbert RE, Stanford M (May 2016). "Antibiotics versus no treatment for toxoplasma retinochoroiditis". The Cochrane Database of Systematic Reviews. 2016 (5): CD002218. doi:10.1002/14651858.CD002218.pub2. PMC 7100541. PMID 27198629.
- ^ "Three-day courses of antibiotics for uncomplicated urinary tract infection | Guidance and guidelines | NICE". 15 January 2015. Archived from the original on 8 December 2015. Retrieved 30 December 2015.
- ^ "TRIMETHOPRIM- trimethoprim tablet". DailyMed. U.S. National Library of Medicine. Archived from the original on 30 September 2015. Retrieved 4 November 2015.
- ^ "PRIMSOL- trimethoprim hydrochloride solution". DailyMed. U.S. National Library of Medicine. Archived from the original on 17 November 2015. Retrieved 4 November 2015.
- ^ "PROLOPRIM® (trimethoprim)100-mg and 200-mg Scored Tablets". DailyMed. U.S. National Library of Medicine. Archived from the original on 17 November 2015. Retrieved 4 November 2015.
- ^ Ellenhorn MJ, Schonwald S, Ordog G, Wasserberger J. American Hospital Formulary Service- Drug Information 2002. Baltimore, MD: Williams and Wilkins. p. 236.
- ^ MICROMEDEX Thomson Health Care. USPDI (2002). Drug Information for the Health Care Professional. Vol. 1 (22nd ed.). Greenwood Village, CO.: Thomson Health Care. p. 2849.
- ^ Choi MJ, Fernandez PC, Patnaik A, Coupaye-Gerard B, D'Andrea D, Szerlip H, et al. (March 1993). "Brief report: trimethoprim-induced hyperkalemia in a patient with AIDS". The New England Journal of Medicine. 328 (10): 703–706. doi:10.1056/NEJM199303113281006. PMID 8433730.
- ^ Naderer O, Nafziger AN, Bertino JS (November 1997). "Effects of moderate-dose versus high-dose trimethoprim on serum creatinine and creatinine clearance and adverse reactions". Antimicrobial Agents and Chemotherapy. 41 (11): 2466–2470. doi:10.1128/AAC.41.11.2466. PMC 164146. PMID 9371351.
- ^ Kimmitt PT, Harwood CR, Barer MR (2000). "Toxin gene expression by shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response". Emerging Infectious Diseases. 6 (5): 458–465. doi:10.3201/eid0605.000503. PMC 2627954. PMID 10998375.
- ^ "PRIMSOL- trimethoprim hydrochloride solution". DailyMed. U.S. National Library of Medicine. Archived from the original on 17 November 2015. Retrieved 4 November 2015.
- ^ Edwards DL, Fink PC, Van Dyke PO (December 1986). "Disulfiram-like reaction associated with intravenous trimethoprim-sulfamethoxazole and metronidazole". Clinical Pharmacy. 5 (12): 999–1000. PMID 3492326. Archived from the original on 24 January 2009.
- ^ Heelon MW, White M (1998). "Disulfiram-cotrimoxazole reaction". Pharmacotherapy. 18 (4): 869–870. doi:10.1002/j.1875-9114.1998.tb03913.x. PMID 9692665. S2CID 23968977. Archived from the original on 24 January 2009.
- ^ Sivojelezova A, Einarson A, Shuhaiber S, Koren G (September 2003). "Trimethoprim-sulfonamide combination therapy in early pregnancy". Canadian Family Physician. 49: 1085–1086. PMC 2214286. PMID 14526858.
- ^ Andersen JT, Petersen M, Jimenez-Solem E, Broedbaek K, Andersen EW, Andersen NL, et al. (August 2013). "Trimethoprim use in early pregnancy and the risk of miscarriage: a register-based nationwide cohort study". Epidemiology and Infection. 141 (8): 1749–1755. doi:10.1017/S0950268812002178. PMC 9151599. PMID 23010291. S2CID 19917493.
- ^ Heaslet H, Harris M, Fahnoe K, Sarver R, Putz H, Chang J, et al. (August 2009). "Structural comparison of chromosomal and exogenous dihydrofolate reductase from Staphylococcus aureus in complex with the potent inhibitor trimethoprim". Proteins. 76 (3): 706–717. doi:10.1002/prot.22383. PMID 19280600. S2CID 1373618.
- ^ a b c d Brogden RN, Carmine AA, Heel RC, Speight TM, Avery GS (June 1982). "Trimethoprim: a review of its antibacterial activity, pharmacokinetics and therapeutic use in urinary tract infections". Drugs. 23 (6): 405–430. doi:10.2165/00003495-198223060-00001. PMID 7049657. S2CID 21806926.
- ^ Burchall JJ (November 1973). "Mechanism of action of trimethoprim-sulfamethoxazole. II". The Journal of Infectious Diseases. 128: Suppl: 437-Suppl: 441. doi:10.1093/infdis/128.Supplement_3.S437. JSTOR 30105875. PMID 4585969.
- ^ Brumfitt W, Hamilton-Miller JM (December 1993). "Reassessment of the rationale for the combinations of sulphonamides with diaminopyrimidines". Journal of Chemotherapy. 5 (6): 465–469. doi:10.1080/1120009X.1993.11741097. PMID 8195839.
