Ocean acidification: Difference between revisions
→Main effects: added an overview summary from an open access publication which is under a compatible licence |
→Main effects: added open access info |
||
| Line 26: | Line 26: | ||
== Main effects == |
== Main effects == |
||
The [[Marine chemistry|ocean’s chemistry]] is changing due to the uptake of anthropogenic carbon dioxide (CO<sub>2</sub>).<ref name=":9">{{Cite journal |last=Jiang |first=Li-Qing |last2=Carter |first2=Brendan R. |last3=Feely |first3=Richard A. |last4=Lauvset |first4=Siv K. |last5=Olsen |first5=Are |date=2019 |title=Surface ocean pH and buffer capacity: past, present and future |url=http://www.nature.com/articles/s41598-019-55039-4 |journal=Scientific Reports |language=en |volume=9 |issue=1 |pages=18624 |doi=10.1038/s41598-019-55039-4 |issn=2045-2322 |pmc=PMC6901524 |pmid=31819102}}</ref> Ocean pH, carbonate ion concentrations ([CO<sub>3</sub><sup>2−</sup>]), and calcium carbonate mineral saturation states (Ω) have been declining as a result of the uptake of approximately 30% of the anthropogenic carbon dioxide emissions over the past 270 years (since around 1750). This process is commonly referred to as “ocean acidification OA”. As the “other CO2 problem”, ocean acidification is making it harder for [[Marine biogenic calcification|marine calcifiers]] to build a shell or skeletal structure, endangering coral reefs and the broader marine ecosystems.<ref name=":9" /> |
The [[Marine chemistry|ocean’s chemistry]] is changing due to the uptake of anthropogenic carbon dioxide (CO<sub>2</sub>).<ref name=":9">{{Cite journal |last=Jiang |first=Li-Qing |last2=Carter |first2=Brendan R. |last3=Feely |first3=Richard A. |last4=Lauvset |first4=Siv K. |last5=Olsen |first5=Are |date=2019 |title=Surface ocean pH and buffer capacity: past, present and future |url=http://www.nature.com/articles/s41598-019-55039-4 |journal=Scientific Reports |language=en |volume=9 |issue=1 |pages=18624 |doi=10.1038/s41598-019-55039-4 |issn=2045-2322 |pmc=PMC6901524 |pmid=31819102|doi-access=free}} [[File:CC-BY icon.svg|50px]] Text was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License]</ref> Ocean pH, carbonate ion concentrations ([CO<sub>3</sub><sup>2−</sup>]), and calcium carbonate mineral saturation states (Ω) have been declining as a result of the uptake of approximately 30% of the anthropogenic carbon dioxide emissions over the past 270 years (since around 1750). This process is commonly referred to as “ocean acidification OA”. As the “other CO2 problem”, ocean acidification is making it harder for [[Marine biogenic calcification|marine calcifiers]] to build a shell or skeletal structure, endangering coral reefs and the broader marine ecosystems.<ref name=":9" /> |
||
=== Reduction in pH value === |
=== Reduction in pH value === |
||
Revision as of 12:17, 16 November 2022
Ocean acidification is the reduction in the pH of the Earth’s ocean. This process takes place over periods lasting decades or more. Its main cause is the absorption of carbon dioxide (CO2) from the atmosphere. This, in turn, increases CO2 concentrations in the ocean. Between 23 and 30% of the CO2 that is in the atmosphere dissolves into oceans, rivers and lakes.[2][3][4] Acidification is one of several effects of rising CO2 on the ocean. Other chemical changes to the ocean can also cause acidification.[5] As the ocean absorbs CO2, seawater chemistry changes, which changes the living conditions of marine species. Many different species are affected, especially organisms that rely on calcium carbonate shells and skeletons, like mollusks, oysters and corals. Organisms like these struggle to build those parts of their anatomy when ocean waters have increased acidity.[6]
When carbon dioxide is absorbed by the ocean, carbonic acid forms and quickly dissociates into a bicarbonate ion (HCO3⁻) and a hydrogen ion (H+). The free hydrogen ions (H+) decrease the ocean pH of the ocean, causing acidification (this does not mean that seawater is acidic yet; it is still alkaline, with a pH higher than 8). The lowered pH causes a decrease in the concentration of carbonate ions, which are the main building block for calcium carbonate (CaCO3) shells and skeletons. It also lowers the carbonate mineral saturation state. Ocean alkalinity is not changed by ocean acidification, but over long time periods alkalinity may increase due to carbonate dissolution and reduced formation of calcium carbonate shells.[7][8]
Between 1751 and 2021, the pH value of the ocean surface is estimated to have decreased from approximately 8.25 to 8.14.[2] This represents an increase of almost 30% in hydrogen ion concentration in the world's oceans (the pH scale is logarithmic, so a change of one in pH unit is equivalent to a tenfold change in hydrogen ion concentration).[9] Sea-surface pH and carbonate saturation states can vary depending on ocean depth and location. Colder and higher latitude waters have the capacity to absorb more CO2. This can increase acidification, lowering the pH and carbonate saturation states in these regions. Other factors that affect the atmosphere-ocean CO2 exchange, and therefore impact local ocean acidification, include: ocean currents (upwelling zones), proximity to large continental rivers, sea ice coverage, and atmospheric exchange with nitrogen and sulfur from fossil fuel burning and agriculture.[10][11][12]
Decreased ocean pH has a range of potentially harmful effects for marine organisms. These include reduced calcification, depressed metabolic rates, lowered immune responses, and reduced energy for basic functions such as reproduction.[13] So the effects of ocean acidification are impacting marine ecosystems that provide food, livelihoods, and other ecosystem services for a large portion of humanity. Some 1 billion people are wholly or partially dependent on the fishing, tourism, and coastal management services provided by coral reefs. Ongoing acidification of the oceans may therefore threaten future food chains linked with the oceans.[7][14]
A statement on ocean acidification by over 100 science academies recommends that by 2050, global CO2 emissions be reduced by at least 50% compared to 1990 levels.[15] The United Nations Sustainable Development Goal 14 ("Life below Water") also has a target to "minimize and address the impacts of ocean acidification".[16]
Ocean acidification has occurred previously in Earth's history. The resulting ecological collapse in the oceans had long-lasting effects on the global carbon cycle and climate.
Cause


2 cycle between the atmosphere and the ocean
Human activities such as the combustion of fossil fuels and land-use changes have led to a new flux of CO
2 into the atmosphere. About 45% has remained in the atmosphere, about 24% has been absorbed by the ocean,[17] and about 32% taken up by land (terrestrial plants).[18]
The carbon cycle describes the fluxes of carbon dioxide (CO
2) between the oceans, terrestrial biosphere, lithosphere,[19] and atmosphere. The carbon cycle involves both organic compounds such as cellulose and inorganic carbon compounds such as carbon dioxide, carbonate ion, and bicarbonate ion, together referenced as dissolved inorganic carbon (DIC). These inorganic compounds are particularly significant in ocean acidification, as they include many forms of dissolved CO
2 present in the Earth's oceans.[20]
When CO
2 dissolves, it reacts with water to form a balance of ionic and non-ionic chemical species: dissolved free carbon dioxide (CO
2(aq)), carbonic acid (H
2CO
3), bicarbonate (HCO−
3) and carbonate (CO2−
3). The ratio of these species depends on factors such as seawater temperature, pressure and salinity (as shown in a Bjerrum plot). These different forms of dissolved inorganic carbon are transferred from an ocean's surface to its interior by the ocean's solubility pump. The resistance of an area of ocean to absorbing atmospheric CO
2 is known as the Revelle factor.
Main effects
The ocean’s chemistry is changing due to the uptake of anthropogenic carbon dioxide (CO2).[21] Ocean pH, carbonate ion concentrations ([CO32−]), and calcium carbonate mineral saturation states (Ω) have been declining as a result of the uptake of approximately 30% of the anthropogenic carbon dioxide emissions over the past 270 years (since around 1750). This process is commonly referred to as “ocean acidification OA”. As the “other CO2 problem”, ocean acidification is making it harder for marine calcifiers to build a shell or skeletal structure, endangering coral reefs and the broader marine ecosystems.[21]
Reduction in pH value
Dissolving CO
2 in seawater increases the hydrogen ion (H+
) concentration in the ocean, and thus decreases ocean pH, as follows:[22]
In shallow coastal and shelf regions, a number of factors interplay to affect air-ocean CO2 exchange and resulting pH change.[23][24] These include biological processes, such as photosynthesis and respiration,[25] as well as water upwelling.[26] Also, ecosystem metabolism in freshwater sources reaching coastal waters can lead to large, but local, pH changes.[23]
Freshwater bodies also appear to be acidifying, although this is a more complex and less obvious phenomenon.[27][28]
Decreased calcification in marine organisms
Changes in ocean chemistry can have extensive direct and indirect effects on organisms and their habitats. One of the most important repercussions of increasing ocean acidity relates to the production of shells out of calcium carbonate (CaCO
3).[29] This process is called calcification and is important to the biology and survival of a wide range of marine organisms. Calcification involves the precipitation of dissolved ions into solid CaCO
3 structures, structures for many marine organisms, such as coccolithophores, foraminifera, crustaceans, mollusks, etc. After they are formed, these CaCO3 structures are vulnerable to dissolution unless the surrounding seawater contains saturating concentrations of carbonate ions (CO32−).
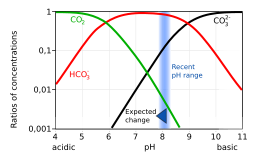
Given the current pH of the ocean (~8.1), of the extra carbon dioxide added into the ocean, very little remains as dissolved carbon dioxide. The majority dissociates into additional bicarbonate and free hydrogen ions. The increase in hydrogen is larger than the increase in bicarbonate,[30] creating an imbalance in the reaction HCO3− ⇌ CO32− + H+. To maintain chemical equilibrium, some of the carbonate ions already in the ocean combine with some of the hydrogen ions to make further bicarbonate. Thus the ocean's concentration of carbonate ions is reduced, removing an essential building block for marine organisms to build shells, or calcify: Ca2+ + CO32− ⇌ CaCO3.
The increase in concentrations of dissolved carbon dioxide and bicarbonate, and reduction in carbonate, are shown in the Bjerrum plot.
Saturation state
The saturation state (known as Ω) of seawater for a mineral is a measure of the thermodynamic potential for the mineral to form or to dissolve, and for calcium carbonate is described by the following equation:
Here Ω is the product of the concentrations (or activities) of the reacting ions that form the mineral (Ca2+ and CO2−3), divided by the apparent solubility product at equilibrium (Ksp), that is, when the rates of precipitation and dissolution are equal..[31] In seawater, a natural horizontal boundary is formed as a result of temperature, pressure, and depth, and is known as the saturation horizon.[29] Above this saturation horizon, Ω has a value greater than 1, and CaCO
3 does not readily dissolve. Most calcifying organisms live in such waters.[29] Below this depth, Ω has a value less than 1, and CaCO
3 will dissolve. The carbonate compensation depth is the ocean depth at which carbonate dissolution balances the supply of carbonate to sea floor, therefore sediment below this depth will be void of calcium carbonate.[32] Increasing CO2 levels, and the resulting lower pH of seawater, decreases the concentration of CO32− and the saturation state of CaCO3 therefore increasing CaCO3 dissolution.
Calcium carbonate most commonly occurs in two common polymorphs (crystalline forms): aragonite and calcite. Aragonite is much more soluble than calcite, so the aragonite saturation horizon, and aragonite compensation depth, is always nearer to the surface than the calcite saturation horizon.[29] This also means that those organisms that produce aragonite may be more vulnerable to changes in ocean acidity than those that produce calcite.[33] Ocean acidification and the resulting decrease in carbonate saturation states raise the saturation horizons of both forms closer to the surface.[34] This decrease in saturation state is one of the main factors leading to decreased calcification in marine organisms because the inorganic precipitation of CaCO3 is directly proportional to its saturation state and calcifying organisms exhibit stress in waters with lower saturation states.[35][36]
Observations and predictions

Observed pH value changes

2 levels between the 1700s and the 1990s, from the Global Ocean Data Analysis Project (GLODAP) and the World Ocean Atlas
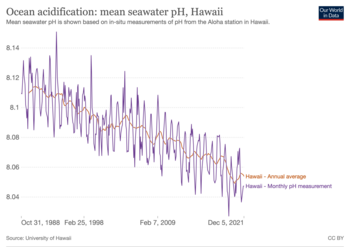
Between 1751 (the beginning of the industrial revolution) and 2021, the pH value of the ocean surface is estimated to have decreased from approximately 8.25 to 8.14.[2][37] This represents an increase of almost 30% in hydrogen ion concentration in the world's oceans (the pH scale is logarithmic, so a change of one in pH unit is equivalent to a tenfold change in hydrogen ion concentration).[9] For example, in the 15-year period 1995–2010 alone, acidity has increased 6 percent in the upper 100 meters of the Pacific Ocean from Hawaii to Alaska.[38]
The rate at which ocean acidification will occur may be influenced by the rate of surface ocean warming, because warm waters will not absorb as much CO2.[39] Therefore, greater seawater warming could limit CO2 absorption and lead to a smaller change in pH for a given increase in CO2.[39] The difference in changes in temperature between basins is one of the main reasons for the differences in acidification rates in different localities. At present, the surface ocean is acidifying at a rate of 0.003-0.026 units per decade. However this rate is faster in the polar regions (-0.002 to -0.026 per decade) than at the subtropical regions (-0.016 to -0.020 per decade)[40]: 83
Current rates of ocean acidification have been likened to the greenhouse event at the Paleocene–Eocene boundary (about 56 million years ago), when surface ocean temperatures rose by 5–6 degrees Celsius. In that event, surface ecosystems experienced a variety of impacts, but bottom-dwelling organisms in the deep ocean actually experienced a major extinction.[41] Currently, the rate of carbon addition to the atmosphere-ocean system is about ten times the rate that occurred at the Paleocene–Eocene boundary.[42]
| Location | Acidification rate (10−3 pH units / year) | Period | Data source |
|---|---|---|---|
| Iceland[43] | -2.4 | 1984 – 2009 | Direct measurements |
| Drake Passage[44] | -1.8 | 2002 – 2012 | Direct measurements |
| Canary (ESTOC)[45] | -1.7 | 1995 – 2004 | Direct measurements |
| Hawaii (HOT)[46] | -1.9 | 1989 – 2007 | Direct measurements |
| Bermuda (BATS)[47] | -1.7 | 1984 – 2012 | Direct measurements |
| Coral Sea[48] | -0.2 | ~1700 – ~1990 | Proxy reconstruction |
| Eastern Mediterranean[49] | -2.3 | 1964 – 2005 | Proxy reconstruction |
Predicted future pH value changes

2 concentration sensor (SAMI-CO2), attached to a Coral Reef Early Warning System station, utilized in conducting ocean acidification studies near coral reef areas (by NOAA (AOML))

2 buoy used for measuring CO
2 concentration and ocean acidification studies (NOAA (by PMEL))
Earth System Models project that, by around 2008, ocean acidity exceeded historical analogues.[50] In combination with other ocean biogeochemical changes, this could undermine the functioning of marine ecosystems and disrupt the provision of many goods and services associated with the ocean beginning as early as 2100.[51]
If the 'business as usual' model for human activity persists (where little effort is made to curb greenhouse gas emissions), model projections estimate that surface ocean pH could decrease by 0.16 to 0.44 units compared to the present day by the end of the century [52]: 608 This represents a further increase in H+ concentrations of two to four times beyond the increase to date.
Current ocean acidification is now on a path to reach lower pH levels than at any other point in the last 300 million years.[53][54] The rate of ocean acidification [55][56] is also estimated to be unprecedented over that same time scale. The expected changes are considered unprecedented in the geological record.[57][58][59]
The impacts of this will be most severe for coral reefs and other shelled marine organisms,[60] as well as those populations that depend on the ecosystem services they provide. The extent of further chemistry changes, including ocean pH, will depend on mitigation efforts taken by nations and their governments.[61]: 704
The largest changes are expected in the future.[33] But already now large quantities of water undersaturated in aragonite are already upwelling close to the Pacific continental shelf area of North America, from Vancouver to Northern California.[62] These continental shelves play an important role in marine ecosystems, since most marine organisms live or are spawned there. Other shelf areas may be experiencing similar effects.[63] In the Mediterranean Sea the strong uptake of anthropogenic CO² is significantly altering the seawater chemistry of surface waters, with measurable pH drops in certain coastal zones.[64]
| Time | pH | pH change relative to pre-industrial |
Source | H+ concentration change relative to pre-industrial |
|---|---|---|---|---|
| Pre-industrial (18th century) | 8.179 | analysed field[65][failed verification] | ||
| Recent past (1990s) | 8.104 | −0.075 | field[65] | + 18.9% |
| Present levels | ~8.069 | −0.11 | field[3][9][66][67] | + 28.8% |
| 2050 (2×CO 2 = 560 ppm) |
7.949 | −0.230 | model[33][failed verification] | + 69.8% |
| 2100 (IS92a)[68] | 7.824 | −0.355 | model[33][failed verification] | + 126.5% |
Ocean acidification in the geologic past
Three of the big five mass extinction events in the geologic past were associated with a rapid increase in atmospheric carbon dioxide, probably due to volcanism and/or thermal dissociation of marine gas hydrates.[69] Elevated CO2 levels impacted biodiversity.[70] Decreased CaCO3 saturation due to seawater uptake of volcanogenic CO2 has been suggested as a possible kill mechanism during the marine mass extinction at the end of the Triassic.[71] The end-Triassic biotic crisis is still the most well-established example of a marine mass extinction due to ocean acidification, because (a) carbon isotope records suggest enhanced volcanic activity that decreased the carbonate sedimentation which reduced the carbonate compensation depth and the carbonate saturation state, and a marine extinction coincided precisely in the stratigraphic record,[72][73][74] and (b) there was pronounced selectivity of the extinction against organisms with thick aragonitic skeletons,[72][75][76] which is predicted from experimental studies.[77] Ocean acidification has also been suggested as a one cause of the end-Permian mass extinction[78][79] and the end-Cretaceous crisis.[80] Overall, multiple climatic stressors, including ocean acidification, was likely the cause of geologic extinction events.[69]
The most notable example of ocean acidification is the Paleocene-Eocene Thermal Maximum (PETM), which occurred approximately 56 million years ago when massive amounts of carbon entered the ocean and atmosphere, and led to the dissolution of carbonate sediments across many ocean basins.[81][82] Relatively new geochemical methods of testing for pH in the past indicate the pH dropped 0.3 units across the PETM.[83][84]One study that solves the marine carbonate system for saturation state shows that it may not change much over the PETM, suggesting the rate of carbon release at our best geological analogy was much slower than human-induced carbon emissions. However, stronger proxy methods to test for saturation state are needed to assess how much this pH change may have affected calcifying organisms.
Impacts on marine life
Ocean acidification has been called the "evil twin of global warming"and "the other CO2 problem".[85][86] Increased ocean temperatures and oxygen loss act concurrently with ocean acidification and constitute the "deadly trio" of climate change pressures on the marine environment.[87]
Increasing ocean acidity many harmful consequences on marine life, such as impacts on oceanic calcifying organisms, other biological impacts, and ecosystem impacts amplified by ocean warming and deoxygenation.
Impacts on oceanic calcifying organisms

Increasing ocean acidification makes it more difficult for shell-accreting organisms to access carbonate ions, essential for the production of their hard exoskeletal shell.[37] Oceanic calcifying organism span the food chain from autotrophs to heterotrophs and include organisms such as coccolithophores, corals, foraminifera, echinoderms, crustaceans and molluscs.[51][88] As described above, under normal conditions, calcite and aragonite are stable in surface waters since the carbonate ions are supersaturated with respect to seawater. However, as ocean pH falls, the concentration of carbonate ions also decreases, and when calcium carbonate becomes undersaturated, structures made of calcium carbonate are vulnerable to calcification stress[89] and dissolution. Therefore, even if there is no change in the rate of calcification, the rate of dissolution of calcareous material increases.[90] In particular, studies show that corals,[91] [92] [93][94] coccolithophores,[88][23] [95]coralline algae,[96] foraminifera,[97] shellfish[82] and pteropods[98] experience reduced calcification or enhanced dissolution when exposed to elevated CO2. A 2010 study from Stony Brook University suggested that even with active marine conservation practices it may be impossible to bring back many previous shellfish populations.[99] Similarly, when exposed in experiments to pH reduced by 0.2 to 0.4, larvae of a temperate brittlestar, a relative of the common sea star, fewer than 0.1 percent survived more than eight days.[51]
The Royal Society published a comprehensive overview of ocean acidification, and its potential consequences, in June 2005.[29] However, some studies have found different responses to ocean acidification, with coccolithophore calcification and photosynthesis both increasing under elevated atmospheric pCO2,[100][101][102] an equal decline in primary production and calcification in response to elevated CO2[103] or the direction of the response varying between species.[104] A study in 2008 examining a sediment core from the North Atlantic found that while the species composition of coccolithophorids has remained unchanged for the industrial period 1780 to 2004, the calcification of coccoliths has increased by up to 40% during the same time.[102] Understanding calcification changes in coccolithophores may have secondary importance because a decline in the coccolithophores may have secondary effects on climate, contributing to global warming by decreasing the Earth's albedo via their effects on oceanic cloud cover.[105] Similarly, the sea star, Pisaster ochraceus, shows enhanced growth in waters with increased acidity[106] Overall, all marine ecosystems on Earth will be exposed to changes in acidification and several other ocean biogeochemical changes.[107] When exposed in experiments to pH reduced by 0.2 to 0.4, larvae of a temperate brittlestar, a relative of the common sea star, fewer than 0.1 percent survived more than eight days.[38] There is also a suggestion that a decline in the coccolithophores may have secondary effects on climate, contributing to global warming by decreasing the Earth's albedo via their effects on oceanic cloud cover.[108] All marine ecosystems on Earth will be exposed to changes in acidification and several other ocean biogeochemical changes.[51]
The fluid in the internal compartments (the coelenteron) where corals grow their exoskeleton is also extremely important for calcification growth. When the saturation rate of aragonite in the external seawater is at ambient levels, the corals will grow their aragonite crystals rapidly in their internal compartments, hence their exoskeleton grows rapidly. If the saturation state of aragonite in the external seawater is lower than the ambient level, the corals have to work harder to maintain the right balance in the internal compartment. When that happens, the process of growing the crystals slows down, and this slows down the rate of how much their exoskeleton is growing. Depending on how much aragonite is in the surrounding water, the corals may even stop growing because the levels of aragonite are too low to pump into the internal compartment. Depending on the aragonite saturation state in the surrounding water, the corals may halt growth because pumping aragonite into the internal compartment will not be energetically favorable.[109] Under the current progression of carbon emissions, around 70% of North Atlantic cold-water corals will be living in corrosive waters by 2050–60.[110]
A study conducted by the Woods Hole Oceanographic Institution in January 2018 showed that acidified conditions primarily reduce the coral’s capacity to build dense exoskeletons, rather than affecting the linear extension of the exoskeleton. Using Global Climate Models, they show that the density of some species of corals could be reduced by over 20% by the end of this century.[111]
An in situ experiment on a 400 m2 patch of the Great Barrier Reef to decrease seawater CO2 level (raise pH) to close to the preindustrial value showed a 7% increase in net calcification.[112] A similar experiment to raise in situ seawater CO2 level (lower pH) to a level expected soon after the middle of this century found that net calcification decreased 34%.[113] However, a field study of the coral reef in Queensland and Western Australia from 2007 to 2012 argues that corals are more resistant to the environmental pH changes than previously thought, due to internal homeostasis regulation; this makes thermal change, which leads to coral bleaching, rather than acidification, the main factor for coral reef vulnerability due to climate change.[114]
Ocean acidification may force some organisms to reallocate resources away from productive endpoints such as growth in order to maintain calcification.[115] For example, the oyster Magallana gigas is recognized to experience metabolic changes alongside altered calcification rates due to energetic tradeoffs resulting from pH imbalances.[116] Therefore, while the full ecological consequences of these changes in calcification are complex, it appears likely that many calcifying species will be adversely affected by ocean acidification.[117]
In some places carbon dioxide bubbles out from the sea floor, locally changing the pH and other aspects of the chemistry of the seawater. Studies of these carbon dioxide seeps have documented a variety of responses by different organisms.[3] Coral reef communities located near carbon dioxide seeps are of particular interest because of the sensitivity of some corals species to acidification. In Papua New Guinea, declining pH caused by carbon dioxide seeps is associated with declines in coral species diversity.[118] However, in Palau carbon dioxide seeps are not associated with reduced species diversity of corals, although bioerosion of coral skeletons is much higher at low pH sites.
Ocean acidification may affect the ocean's biologically driven sequestration of carbon from the atmosphere to the ocean interior and seafloor sediment, weakening the so-called biological pump.[119] Seawater acidification could also reduce the size of Antarctic phytoplankton, making them less effective at storing carbon.[120] Such changes are being increasingly studied and synthesized through the use of physiological frameworks, including the Adverse Outcome Pathway (AOP) framework.[116]
Warm water corals are clearly in decline, with losses of 50% over the last 30-50 years due to multiple threats from ocean warming, ocean acidification, pollution and physical damage from activities such as fishing, and these pressures are expected to intensify.[121][122]: 416
Other biological impacts
Aside from the slowing and/or reversal of calcification, organisms may suffer other adverse effects, either indirectly through negative impacts on food resources,[29] or directly as reproductive or physiological effects. For example, the elevated oceanic levels of CO2 may produce CO
2-induced acidification of body fluids, known as hypercapnia. Also, increasing ocean acidity is believed to have a range of direct consequences. For example, increasing acidity has been observed to: reduce metabolic rates in jumbo squid;[91] depress the immune responses of blue mussels.[92] This is possibly because ocean acidification may alter the acoustic properties of seawater, allowing sound to propagate further, and increasing ocean noise.[123] This impacts all animals that use sound for echolocation or communication.[124] Atlantic longfin squid eggs took longer to hatch in acidified water, and the squid's statolith was smaller and malformed in animals placed in sea water with a lower pH.[125] However, these studies are ongoing and there is not a full understanding of these processes in marine organisms or ecosystems.[126]
Another possible effect would be an increase in red tide events, which could contribute to the accumulation of toxins (domoic acid, brevetoxin, saxitoxin) in small organisms such as anchovies and shellfish, in turn increasing occurrences of amnesic shellfish poisoning, neurotoxic shellfish poisoning and paralytic shellfish poisoning.[127]
Although red tide is harmful, other beneficial photosynthetic organisms may benefit from increased levels of carbon dioxide. Most importantly, seagrasses will benefit.[128] An experiment done in 2018 concluded that as seagrasses increased their photosynthetic activity, calcifying algae's calcification rates rose. This could be a potential mitigation technique in the face of increasing acidity.[128]
Fish larvae
Ocean acidification can also have affects on marine fish larvae. It internally affects their olfactory systems, which is a crucial part of their development, especially in the beginning stage of their life. Orange clownfish larvae mostly live on oceanic reefs that are surrounded by vegetative islands.[129] With the use of their sense of smell, larvae are known to be able to detect the differences between reefs surrounded by vegetative islands and reefs not surrounded by vegetative islands.[129] Clownfish larvae need to be able to distinguish between these two destinations to have the ability to locate an area that is satisfactory for their growth. Another use for marine fish olfactory systems is to help in determining the difference between their parents and other adult fish in order to avoid inbreeding.
At James Cook University's experimental aquarium facility, clownfish were sustained in non-manipulated seawater that obtained a pH of 8.15 ± 0.07 which is similar to our current ocean's pH. To test for effects of different pH levels, seawater was manipulated to three different pH levels, including the non-manipulated pH. The two opposing pH levels correspond with climate change models that predict future atmospheric CO2 levels.[129] In the year 2100 the model predicts that we could potentially acquire CO2 levels at 1,000 ppm, which correlates with the pH of 7.8 ± 0.05. Results of this experiment show that when larvae is exposed to a pH of 7.8 ± 0.05 their reaction to environmental cues differs drastically to larvae's reaction to cues in a non-manipulated pH. At the pH of 7.6 ± 0.05 larvae had no reaction to any type of cue. However, a meta-analysis published in 2022 found that the effect sizes of published studies testing for ocean acidification effects on fish behavior have declined by an order of magnitude over the past decade and have been negligible for the past five years.[130]
Ecosystem impacts amplified by ocean warming and deoxygenation

While the full implications of elevated CO2 on marine ecosystems are still being documented, there is a substantial body of research showing that a combination of ocean acidification and elevated ocean temperature, driven mainly by CO2 and other greenhouse gas emissions, have a compounded effect on marine life and the ocean environment. This effect far exceeds the individual harmful impact of either.[133][134][135] In addition, ocean warming, along with increased productivity of phytoplankton from higher CO2 levels exacerbates ocean deoxygenation. Deoxygenation of ocean waters is an additional stressor on marine organisms that increases ocean stratification therefore limiting nutrients over time and reducing biological gradients.[136][137]
Meta analyses have quantified the direction and magnitude of the harmful effects of ocean acidification, warming and deoxygenation on the ocean.[138][139][140] These meta-analyses have been further tested by mesocosm studies[141][142] that simulated the interaction of these stressors and found a catastrophic effect on the marine food web, i.e. that the increases in consumption from thermal stress more than negates any primary producer to herbivore increase from elevated CO2.
Impacts on the economy and societies
The increase of ocean acidity decelerates the rate of calcification in salt water, leading to smaller and slower growing coral reefs which supports approximately 25% of marine life.[143][144] Impacts are far-reaching from fisheries and coastal environments down to the deepest depths of the ocean.[145] The increase in ocean acidity in not only killing the coral, but also the wildly diverse population of marine inhabitants which coral reefs support.[146]
Fishing industry
The threat of acidification includes a decline in commercial fisheries and in the Arctic tourism industry and economy. Commercial fisheries are threatened because acidification harms calcifying organisms which form the base of the Arctic food webs.
Pteropods and brittle stars both form the base of the Arctic food webs and are both seriously damaged from acidification. Pteropods shells dissolve with increasing acidification and the brittle stars lose muscle mass when re-growing appendages.[147] For pteropods to create shells they require aragonite which is produced through carbonate ions and dissolved calcium and strontium. Pteropods are severely affected because increasing acidification levels have steadily decreased the amount of water supersaturated with carbonate.[148] The degradation of organic matter in Arctic waters has amplified ocean acidification; some Arctic waters are already undersaturated with respect to aragonite.[148] In the North Pacific and North Atlantic, saturation states are also decreasing (the depth of saturation is getting more shallow). Additionally the brittle star's eggs die within a few days when exposed to expected conditions resulting from Arctic acidification.[149]
Acidification threatens to destroy Arctic food webs from the base up. Arctic food webs are considered simple, meaning there are few steps in the food chain from small organisms to larger predators. For example, pteropods are "a key prey item of a number of higher predators – larger plankton, fish, seabirds, whales".[150] Both pteropods and sea stars serve as a substantial food source and their removal from the simple food web would pose a serious threat to the whole ecosystem. The effects on the calcifying organisms at the base of the food webs could potentially destroy fisheries. The value of fish caught from US commercial fisheries in 2007 was valued at $3.8 billion and of that 73% was derived from calcifiers and their direct predators.[151] Other organisms are directly harmed as a result of acidification. For example, decrease in the growth of marine calcifiers such as the American lobster, ocean quahog, and scallops means there is less shellfish meat available for sale and consumption.[152] Red king crab fisheries are also at a serious threat because crabs are calcifiers and rely on carbonate ions for shell development. Baby red king crab when exposed to increased acidification levels experienced 100% mortality after 95 days.[153] In 2006, red king crab accounted for 23% of the total guideline harvest levels and a serious decline in red crab population would threaten the crab harvesting industry.[154] Several ocean goods and services are likely to be undermined by future ocean acidification potentially affecting the livelihoods of some 400 to 800 million people depending upon the emission scenario.[51]
Indigenous peoples
Acidification will affect the way of life of indigenous peoples. Sport fishing and hunting are both culturally important to Arctic Indigenous peoples.The rapid decrease or disappearance of marine life could also affect the diet of Indigenous peoples. For example, in Washington State and California (United States), Native American communities report potential damage to shellfish resources due to sea level rise and ocean acidification.[155]
Other impacts
At depths of 1000s of meters in the ocean, calcium carbonate shells begin to dissolve as increasing pressure and decreasing temperature shift the chemical equilibria controlling calcium carbonate precipitation.[156] The depth at which this occurs is known as the carbonate compensation depth. Ocean acidification will increase such dissolution and shallow the carbonate compensation depth on timescales of tens to hundreds of years.[156] Zones of downwelling are being affected first.[157]
It is expected that ocean acidification in the future will lead to a significant decrease in the burial of carbonate sediments for several centuries, and even the dissolution of existing carbonate sediments.[158] This will cause an elevation of ocean alkalinity, leading to the enhancement of the ocean as a reservoir for CO2, which would cause further invasion of CO2 from the atmosphere to the ocean.[159]
Possible responses

Reducing greenhouse gas emissions
Given that ocean acidification is caused by anthropogenic carbon dioxide emissions, the number one ocean acidification mitigation strategy is to reduce these emissions. In 2009, members of the InterAcademy Panel called on world leaders to "Recognize that reducing the build up of CO2 in the atmosphere is the only practicable solution to mitigating ocean acidification".[15] The statement also stressed the importance to "Reinvigorate action to reduce stressors, such as overfishing and pollution, on marine ecosystems to increase resilience to ocean acidification".[160]
Increasing the land devoted to forests and encouraging the growth of CO2-breathing sea plants can mitigate ocean acidification.[161]
Technologies to remove carbon dioxide from the ocean
Intervention and mitigation approaches that remove carbon dioxide from the ocean, known as carbon dioxide removal (CDR), include ocean nutrient fertilization, artificial upwelling/downwelling, seaweed farming, ecosystem recovery, ocean alkalinity enhancement, and electrochemical processes. All of these methods use the ocean to remove CO2 from the atmosphere to store it in the ocean. However, a few of the methods have an additional positive effect, or a co-benefit, of mitigating ocean acidification. The research field for all CDR methods has grown tremendously since 2019.[162]
Ocean nutrient fertilization
Ocean fertilization or ocean nourishment is a type of technology for carbon dioxide removal from the ocean based on the purposeful introduction of plant nutrients to the upper ocean to increase marine food production and to remove carbon dioxide from the atmosphere.[163][164] Ocean nutrient fertilization, for example iron fertilization, could stimulate photosynthesis in phytoplankton. The phytoplankton would convert the ocean's dissolved carbon dioxide into carbohydrate, some of which would sink into the deeper ocean before oxidizing. More than a dozen open-sea experiments confirmed that adding iron to the ocean increases photosynthesis in phytoplankton by up to 30 times.[165]
This is one of the more well-researched carbon dioxide removal (CDR) approaches, however this approach would only sequester carbon on a timescale of 10–100 years[clarification needed] dependent on ocean mixing times. While surface ocean acidity may decrease as a result of nutrient fertilization, when the sinking organic matter remineralizes, deep ocean acidity will increase. A 2021 report on CDR indicates that there is medium-high confidence that the technique could be efficient and scalable at low cost, with medium environmental risks.[166] One of the key risks of nutrient fertilization is nutrient robbing, a process by which excess nutrients used in one location for enhanced primary productivity, as in a fertilization context, are then unavailable for normal productivity downstream.[clarification needed] This could result in ecosystem impacts far outside the original site of fertilization.[166]Ocean alkalinity enhancement
Ocean alkalinity enhancement (OAE) is the process of accelerating Earth’s geologic carbon regulator. The process involves increasing the amount of bicarbonate (HCO3-) through accelerated weathering of rocks (silicate, limestone and quicklime).[162] This process mimics the silicate-carbonate cycle, and will ultimately draw down CO2 from the atmosphere, into the ocean. The CO2 will either become bicarbonate, and be stored in the ocean in that form for >100 years, or may precipitate into CaCO3, which when buried in the deep ocean, can store the carbon for ~1 million years when utilizing silicate rocks as the means to increase alkalinity. In addition to sequestering CO2, alkalinity addition buffers the pH of the ocean therefore mitigating ocean acidification. However, little is known about how organisms will respond to added alkalinity, even from natural sources.[162] For example, weathering of some silicate rocks could release a large amount of potentially trace metals into the ocean at the site of enhanced weathering. In addition, the cost and the energy consumed by implementing ocean alkalinity enhancement (mining, pulverizing, transport) is high compared to other CDR techniques. Overall, OAE is scalable, and highly efficient at removing carbon dioxide.[162]
Electrochemical processes
Electrochemical methods, or electrolysis, can strip carbon dioxide directly from seawater.[162] Some methods focus on direct CO2 removal (in the form of carbonate and CO2 gas) while others increase the alkalinity of seawater by precipitating metal hydroxide residues, which absorbs CO2 in a matter described in the ocean alkalinity enhancement section. The hydrogen produced during direct carbon capture can then be upcycled to form hydrogen for energy consumption, or other manufactured laboratory reagents such as hydrochloric acid. Electrolysis is a classic chemical technique that dates back to the 19th century. However, implementation of electrolysis for carbon capture is expensive and the energy consumed for the process is high compared to other CDR techniques.[162] In addition, research to assess the environmental impact of this process is ongoing. Some complications include toxic chemicals in wastewaters, and reduced DIC in effluents; both of these may negatively impact marine life. Similar to OAE, recent reports show electrochemical processes are scalable and highly efficient at removing carbon dioxide.[162]
Policies and goals
Global policies
As awareness about ocean acidification grows, policies geared towards increasing monitoring efforts of ocean acidification have been drafted.[167] International efforts, such as the UN Cartagena Convention,[168] are critical to enhance the support provided by regional governments to highly vulnerable areas to ocean acidification. Many countries, for example in the Pacific Islands and Territories, have constructed regional policies, or National Ocean Policies, National Action Plans, National Adaptation Plans of Action and Joint National Action Plans on Climate Change and Disaster Risk Reduction, to help work towards SDG 14. Ocean acidification is now starting to be considered within those frameworks.[169]
UN Ocean Decade
The UN Ocean Decade Action "OARS: Ocean Acidification Research for Sustainability” was proposed by the Global Ocean Acidification Observing Network (GOA-ON) and its partners, and has been formally endorsed as a program of the UN Decade of Ocean Science for Sustainable Development.[170][171] The OARS programme builds on the work of GOA-ON to further develop the science of ocean acidification by enhancing ocean acidification capacity, increasing observations of ocean chemistry changes, identifying the impacts on marine ecosystems on local and global scales, and providing society and decision makers with the information needed to mitigate and adapt to ocean acidification.
Global Climate Indicators
The importance of ocean acidification is reflected in its inclusion as one of seven Global Climate Indicators.[172] These Indicators are a set of parameters that describe the changing climate without reducing climate change to only rising temperature. The Indicators include key information for the most relevant domains of climate change: temperature and energy, atmospheric composition, ocean and water as well as the cryosphere. The Global Climate Indicators have been identified by scientists and communication specialists in a process led by Global Climate Observing System (GCOS).[173] The Indicators have been endorsed by the World Meteorological Organization (WMO). They form the basis of the annual WMO Statement of the State of the Global Climate, which is submitted to the Conference of Parties (COP) of the United Nations Framework Convention on Climate Change (UNFCCC). Additionally, the Copernicus Climate Change Service (C3S) of the European Commission uses the Indicators for their annual "European State of the Climate".
Sustainable Development Goal 14
In 2015, the United Nations adopted the 2030 Agenda and a set of 17 Sustainable Development Goals (SDG), including a goal dedicated to the ocean, Sustainable Development Goal 14,[16] which calls to "conserve and sustainably use the oceans, seas and marine resources for sustainable development". Ocean acidification is directly addressed by the target SDG 14.3. The full title of Target 14.3 is: "Minimize and address the impacts of ocean acidification, including through enhanced scientific cooperation at all levels".[174] This target has one indicator: Indicator 14.3.1 which calls for the "Average marine acidity (pH) measured at agreed suite of representative sampling stations".[175]
The Intergovernmental Oceanographic Commission (IOC) of UNESCO was identified as the custodian agency for the SDG 14.3.1 Indicator. In this role, IOC-UNESCO is tasked with developing the SDG 14.3.1 Indicator Methodology, the annual collection of data towards the SDG 14.3.1 Indicator and the reporting of progress to the United Nations.[176][177]
Policies at country level
United States
In the United States, robust ocean acidification policy[178] supports sustained government coordination, such as the National Oceanic Atmospheric Administration’s Ocean Acidification Program.[179] In 2015, USEPA denied a citizens petition that asked EPA to regulate CO2 under the Toxic Substances Control Act of 1976 in order to mitigate ocean acidification.[180][181] In the denial, the EPA said that risks from ocean acidification were being "more efficiently and effectively addressed" under domestic actions, e.g., under the Presidential Climate Action Plan,[182] and that multiple avenues are being pursued to work with and in other nations to reduce emissions and deforestation and promote clean energy and energy efficiency.
See also
- Biological pump – Carbon capture process in oceans
- Free Ocean CO2 Enrichment - technology for studying ocean acidification
- Carbon sink – Reservoir absorbing more carbon from, than emitting to, the air
- Estuarine acidification – Reducing pH values in coastal marine ecosystems
- Holocene extinction – Ongoing extinction event caused by human activity
- Ocean acidification in the Arctic Ocean
- Ocean acidification in the Great Barrier Reef – Threat to the reef which reduces the viability and strength of reef-building corals
- Ocean deoxygenation – Reduction of the oxygen content of the oceans
- Ocean storage of carbon dioxide – Storing carbon in a carbon pool (natural as well as enhanced or artificial processes)
- Water pollution
References
- ^ Ritchie, Roser, Mispy, Ortiz-Ospina. "SDG 14 - Measuring progress towards the Sustainable Development Goals." SDG-Tracker.org, website (2018).
- ^ a b c Jacobson, M. Z. (2005). "Studying ocean acidification with conservative, stable numerical schemes for nonequilibrium air-ocean exchange and ocean equilibrium chemistry". Journal of Geophysical Research: Atmospheres. 110: D07302. Bibcode:2005JGRD..11007302J. doi:10.1029/2004JD005220.
- ^ a b c Hall-Spencer, J. M.; Rodolfo-Metalpa, R.; Martin, S.; et al. (July 2008). "Volcanic carbon dioxide vents show ecosystem effects of ocean acidification". Nature. 454 (7200): 96–9. Bibcode:2008Natur.454...96H. doi:10.1038/nature07051. hdl:10026.1/1345. PMID 18536730. S2CID 9375062.
- ^ Friedlingstein, Pierre; O'Sullivan, Michael; Jones, Matthew W.; Andrew, Robbie M.; Hauck, Judith; Olsen, Are; Peters, Glen P.; Peters, Wouter; Pongratz, Julia; Sitch, Stephen; Le Quéré, Corinne; Canadell, Josep G.; Ciais, Philippe; Jackson, Robert B.; Alin, Simone (11 December 2020). "Global Carbon Budget 2020". Earth System Science Data. 12 (4): 3269–3340. Bibcode:2020ESSD...12.3269F. doi:10.5194/essd-12-3269-2020. ISSN 1866-3508.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Field, C.B.; Barros, V; Stocker, T.F.; Dahe, Q; et al. "IPCC Workshop on Ocean Acidification on Marine Biology and Ecosystems — IPCC". IPCC Workshop on Ocean Acidification on Marine Biology and Ecosystems. Retrieved 28 September 2022.
{{cite web}}: CS1 maint: url-status (link) - ^ Robert E. Service (13 July 2012). "Rising Acidity Brings and Ocean Of Trouble". Science. 337 (6091): 146–148. Bibcode:2012Sci...337..146S. doi:10.1126/science.337.6091.146. PMID 22798578.
- ^ a b Cornelia Dean (30 January 2009). "Rising Acidity Is Threatening Food Web of Oceans, Science Panel Says". New York Times.
- ^ Boudreau, Bernard P.; Middelburg, Jack J.; Luo, Yiming (30 November 2018). "The role of calcification in carbonate compensation". Nature Geoscience. 11 (12): 894–900. Bibcode:2018NatGe..11..894B. doi:10.1038/s41561-018-0259-5. ISSN 1752-0908. S2CID 135284130.
- ^ a b c "Report of the Ocean Acidification and Oxygen Working Group, International Council for Science's Scientific Committee on Ocean Research (SCOR) Biological Observatories Workshop" (PDF).
- ^ Osborne, Emily B.; Thunell, Robert C.; Gruber, Nicolas; Feely, Richard A.; Benitez-Nelson, Claudia R. (16 December 2019). "Decadal variability in twentieth-century ocean acidification in the California Current Ecosystem". Nature Geoscience. 13 (1): 43–49. doi:10.1038/s41561-019-0499-z. ISSN 1752-0908. S2CID 209381004.
- ^ Wallace, Ryan B.; Baumann, Hannes; Grear, Jason S.; Aller, Robert C.; Gobler, Christopher J. (5 July 2014). "Coastal ocean acidification: The other eutrophication problem". Estuarine, Coastal and Shelf Science. 148: 1–13. Bibcode:2014ECSS..148....1W. doi:10.1016/j.ecss.2014.05.027. ISSN 0272-7714.
- ^ Doney, Scott C.; Mahowald, Natalie; Lima, Ivan; Feely, Richard A.; Mackenzie, Fred T.; Lamarque, Jean-Francois; Rasch, Phil J. (11 September 2007). "Impact of anthropogenic atmospheric nitrogen and sulfur deposition on ocean acidification and the inorganic carbon system". Proceedings of the National Academy of Sciences. 104 (37): 14580–14585. Bibcode:2007PNAS..10414580D. doi:10.1073/pnas.0702218104. ISSN 0027-8424. PMC 1965482. PMID 17804807.
- ^ Anthony, KRN; et al. (2008). "Ocean acidification causes bleaching and productivity loss in coral reef builders". Proceedings of the National Academy of Sciences. 105 (45): 17442–17446. Bibcode:2008PNAS..10517442A. doi:10.1073/pnas.0804478105. PMC 2580748. PMID 18988740.
- ^ Robert E. Service (13 July 2012). "Rising Acidity Brings and Ocean Of Trouble". Science. 337 (6091): 146–148. Bibcode:2012Sci...337..146S. doi:10.1126/science.337.6091.146. PMID 22798578.
- ^ a b IAP (June 2009). "Interacademy Panel (IAP) Member Academies Statement on Ocean Acidification"., Secretariat: TWAS (the Academy of Sciences for the Developing World), Trieste, Italy.
- ^ a b "Goal 14 targets". UNDP. Retrieved 24 September 2020.
- ^ Raven, J. A.; Falkowski, P. G. (1999). "Oceanic sinks for atmospheric CO2". Plant, Cell & Environment. 22 (6): 741–755. doi:10.1046/j.1365-3040.1999.00419.x.
- ^ Friedlingstein, Pierre; Jones, Matthew W.; O'Sullivan, Michael; Andrew, Robbie M.; et al. (26 April 2022). "Global Carbon Budget 2021". Earth System Science Data. 14 (4): 1917–2005. Bibcode:2022ESSD...14.1917F. doi:10.5194/essd-14-1917-2022. ISSN 1866-3508.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "carbon cycle". Encyclopædia Britannica Online. Retrieved 11 February 2010.
- ^ Kump, Lee R.; Kasting, James F.; Crane, Robert G. (2003). The Earth System (2nd ed.). Upper Saddle River: Prentice Hall. pp. 162–164. ISBN 978-0-613-91814-5.
- ^ a b Jiang, Li-Qing; Carter, Brendan R.; Feely, Richard A.; Lauvset, Siv K.; Olsen, Are (2019). "Surface ocean pH and buffer capacity: past, present and future". Scientific Reports. 9 (1): 18624. doi:10.1038/s41598-019-55039-4. ISSN 2045-2322. PMC 6901524. PMID 31819102.
{{cite journal}}: CS1 maint: PMC format (link) Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
- ^ Paul Freund; Stefan Bachu; Dale Simbeck; Kelly (Kailai) Thambimuthu; Murlidhar Gupta (2005). "Annex I: Properties of CO2 and carbon-based fuels". In Bert Metz; Ogunlade Davidson; Heleen de Coninck; Manuela Loos; Leo Meyer (eds.). IPCC Special Report on Carbon Dioxide Capture and Storage (PDF). IPCC. p. 390. Archived from the original (PDF) on 10 February 2010. Retrieved 1 November 2014.
- ^ a b c Carstensen, Jacob; Duarte, Carlos M. (16 April 2019). "Drivers of pH Variability in Coastal Ecosystems". Environmental Science & Technology. 53 (8): 4020–4029. Bibcode:2019EnST...53.4020C. doi:10.1021/acs.est.8b03655. ISSN 0013-936X. PMID 30892892. S2CID 84841808.
- ^ Duarte, Carlos M.; Hendriks, Iris E.; Moore, Tommy S.; Olsen, Ylva S.; Steckbauer, Alexandra; Ramajo, Laura; Carstensen, Jacob; Trotter, Julie A.; McCulloch, Malcolm (1 March 2013). "Is Ocean Acidification an Open-Ocean Syndrome? Understanding Anthropogenic Impacts on Seawater pH". Estuaries and Coasts. 36 (2): 221–236. doi:10.1007/s12237-013-9594-3. ISSN 1559-2731.
- ^ Lowe, Alexander T.; Bos, Julia; Ruesink, Jennifer (30 January 2019). "Ecosystem metabolism drives pH variability and modulates long-term ocean acidification in the Northeast Pacific coastal ocean". Scientific Reports. 9 (1): 963. Bibcode:2019NatSR...9..963L. doi:10.1038/s41598-018-37764-4. ISSN 2045-2322. PMC 6353961. PMID 30700764.
- ^ Fairchild, William; Hales, Burke (2021). "High-Resolution Carbonate System Dynamics of Netarts Bay, OR From 2014 to 2019". Frontiers in Marine Science. 7. doi:10.3389/fmars.2020.590236. ISSN 2296-7745.
- ^ Gies, E. (11 January 2018). "Like Oceans, Freshwater Is Also Acidifying". Scientific American. Retrieved 13 January 2018.
- ^ Weiss, L. C.; Pötter, L.; Steiger, A.; Kruppert, S.; Frost, U.; Tollrian, R. (2018). "Rising pCO2 in Freshwater Ecosystems Has the Potential to Negatively Affect Predator-Induced Defenses in Daphnia". Current Biology. 28 (2): 327–332.e3. doi:10.1016/j.cub.2017.12.022. PMID 29337079.
- ^ a b c d e f Raven, JA, et al. (2005) "Ocean acidification due to increasing atmospheric carbon dioxide". Royal Society, London, UK.
- ^ Mitchell, M. J.; et al. (2010). "A model of carbon dioxide dissolution and mineral carbonation kinetics". Proceedings of the Royal Society A. 466 (2117): 1265–1290. Bibcode:2010RSPSA.466.1265M. doi:10.1098/rspa.2009.0349.
- ^ Atkinson, M.J.; Cuet, P. (2008). "Possible effects of ocean acidification on coral reef biogeochemistry: topics for research". Marine Ecology Progress Series. 373: 249–256. Bibcode:2008MEPS..373..249A. doi:10.3354/meps07867.
- ^ Thurman, H.V.; Trujillo, A.P. (2004). Introductory Oceanography. Prentice Hall. ISBN 978-0-13-143888-0.
- ^ a b c d e Orr, James C.; et al. (2005). "Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms" (PDF). Nature. 437 (7059): 681–686. Bibcode:2005Natur.437..681O. doi:10.1038/nature04095. PMID 16193043. S2CID 4306199. Archived from the original (PDF) on 25 June 2008.
- ^ The Royal Society. Ocean Acidification Due To Increasing Atmospheric Carbon Dioxide, The Clyvedon Press Ltd. (2005): 11.
- ^ Marubini, F.; Ferrier-Pagès, C.; Furla, P.; Allemand, D. (2008). "Coral calcification responds to seawater acidification: a working hypothesis towards a physiological mechanism". Coral Reefs. 27 (3): 491–499. Bibcode:2008CorRe..27..491M. doi:10.1007/s00338-008-0375-6.
- ^ Gattuso, Jean-Pierre; Mach, Katharine J.; Morgan, Granger (2013). "Ocean acidification and its impacts: An expert survey". Climatic Change. 117 (4): 725–738. Bibcode:2013ClCh..117..725G. doi:10.1007/s10584-012-0591-5. S2CID 153892043.
- ^ a b "PMEL CO2 - Carbon Dioxide Program". www.pmel.noaa.gov. Retrieved 6 September 2021.
- ^ a b Marah J. Hardt; Carl Safina (9 August 2010). "How Acidification Threatens Oceans from the Inside Out". Scientific American. Archived from the original on 26 December 2010.
- ^ a b Humphreys, M. P. (2016). "Climate sensitivity and the rate of ocean acidification: future impacts, and implications for experimental design". ICES Journal of Marine Science. 74 (4): 934–940. doi:10.1093/icesjms/fsw189.
- ^ Gulev, S.K., P.W. Thorne, J. Ahn, F.J. Dentener, C.M. Domingues, S. Gerland, D. Gong, D.S. Kaufman, H.C. Nnamchi, J. Quaas, J.A. Rivera, S. Sathyendranath, S.L. Smith, B. Trewin, K. von Schuckmann, and R.S. Vose, 2021: Changing State of the Climate System. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 287–422, doi:10.1017/9781009157896.004.
- ^ McInerney, Francesca A.; Wing, Scott L. (30 May 2011). "The Paleocene-Eocene Thermal Maximum: A Perturbation of Carbon Cycle, Climate, and Biosphere with Implications for the Future". Annual Review of Earth and Planetary Sciences. 39 (1): 489–516. Bibcode:2011AREPS..39..489M. doi:10.1146/annurev-earth-040610-133431. ISSN 0084-6597.
- ^ Zeebe, Richard E. (30 May 2012). "History of Seawater Carbonate Chemistry, Atmospheric CO 2 , and Ocean Acidification". Annual Review of Earth and Planetary Sciences. 40 (1): 141–165. Bibcode:2012AREPS..40..141Z. doi:10.1146/annurev-earth-042711-105521. ISSN 0084-6597.
- ^ Olafsson, J.; Olafsdottir, S. R.; Benoit-Cattin, A.; Danielsen, M.; Arnarson, T. S.; Takahashi, T. (25 November 2009). "Rate of Iceland Sea acidification from time series measurements". Biogeosciences. 6 (11): 2661–2668. Bibcode:2009BGeo....6.2661O. doi:10.5194/bg-6-2661-2009.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Midorikawa, Takashi; Inoue, Hisayuki Y.; Ishii, Masao; Sasano, Daisuke; Kosugi, Naohiro; Hashida, Gen; Nakaoka, Shin-ichiro; Suzuki, Toru (March 2012). "Decreasing pH trend estimated from 35-year time series of carbonate parameters in the Pacific sector of the Southern Ocean in summer". Deep Sea Research Part I: Oceanographic Research Papers. 61: 131–139. Bibcode:2012DSRI...61..131M. doi:10.1016/j.dsr.2011.12.003.
- ^ González-Dávila, M.; Santana-Casiano, J. M.; Rueda, M. J.; Llinás, O. (11 October 2010). "The water column distribution of carbonate system variables at the ESTOC site from 1995 to 2004". Biogeosciences. 7 (10): 3067–3081. Bibcode:2010BGeo....7.3067G. doi:10.5194/bg-7-3067-2010.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Dore, J. E.; Lukas, R.; Sadler, D. W.; Church, M. J.; Karl, D. M. (28 July 2009). "Physical and biogeochemical modulation of ocean acidification in the central North Pacific". Proceedings of the National Academy of Sciences. 106 (30): 12235–12240. doi:10.1073/pnas.0906044106. PMC 2716384. PMID 19666624.
- ^ Bates, N. R.; Best, M. H. P.; Neely, K.; Garley, R.; Dickson, A. G.; Johnson, R. J. (11 July 2012). "Detecting anthropogenic carbon dioxide uptake and ocean acidification in the North Atlantic Ocean". Biogeosciences. 9 (7): 2509–2522. Bibcode:2012BGeo....9.2509B. doi:10.5194/bg-9-2509-2012.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Pelejero, Carles; Calvo, Eva; McCulloch, Malcolm T.; Marshall, John F.; Gagan, Michael K.; Lough, Janice M.; Opdyke, Bradley N. (30 September 2005). "Preindustrial to Modern Interdecadal Variability in Coral Reef pH". Science. 309 (5744): 2204–2207. Bibcode:2005Sci...309.2204P. doi:10.1126/science.1113692. PMID 16195458. S2CID 129883047.
- ^ Bialik, Or M.; Sisma-Ventura, Guy (December 2016). "Proxy-based reconstruction of surface water acidification and carbonate saturation of the Levant Sea during the Anthropocene". Anthropocene. 16: 42–53. doi:10.1016/j.ancene.2016.08.001.
- ^ Mora, Camilo; Frazier, Abby G.; Longman, Ryan J.; Dacks, Rachel S.; Walton, Maya M.; Tong, Eric J.; Sanchez, Joseph J.; Kaiser, Lauren R.; Stender, Yuko O.; Anderson, James M.; Ambrosino, Christine M. (10 October 2013). "The projected timing of climate departure from recent variability". Nature. 502 (7470): 183–187. Bibcode:2013Natur.502..183M. doi:10.1038/nature12540. ISSN 0028-0836. PMID 24108050. S2CID 4471413.
- ^ a b c d e Mora, Camilo; Wei, Chih-Lin; Rollo, Audrey; Amaro, Teresa; Baco, Amy R.; Billett, David; Bopp, Laurent; Chen, Qi; Collier, Mark; Danovaro, Roberto; Gooday, Andrew J.; Grupe, Benjamin M.; Halloran, Paul R.; Ingels, Jeroen; Jones, Daniel O. B. (15 October 2013). Mace, Georgina M. (ed.). "Biotic and Human Vulnerability to Projected Changes in Ocean Biogeochemistry over the 21st Century". PLOS Biology. 11 (10): e1001682. doi:10.1371/journal.pbio.1001682. ISSN 1545-7885. PMC 3797030. PMID 24143135.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Lee, J.-Y., J. Marotzke, G. Bala, L. Cao, S. Corti, J.P. Dunne, F. Engelbrecht, E. Fischer, J.C. Fyfe, C. Jones, A. Maycock, J. Mutemi, O. Ndiaye, S. Panickal, and T. Zhou, 2021: Future Global Climate: Scenario-Based Projections and Near- Term Information. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 553–672, doi:10.1017/9781009157896.006.
- ^ Joel, Lucas (21 October 2019). "The Dinosaur-Killing Asteroid Acidified the Ocean in a Flash - The Chicxulub event was as damaging to life in the oceans as it was to creatures on land, a study shows". The New York Times. Retrieved 22 October 2019.
- ^ Henehan, Michael J.; et al. (21 October 2019). "Rapid ocean acidification and protracted Earth system recovery followed the end-Cretaceous Chicxulub impact". Proceedings of the National Academy of Sciences of the United States of America. 116 (45): 22500–22504. Bibcode:2019PNAS..11622500H. doi:10.1073/pnas.1905989116. PMC 6842625. PMID 31636204.
- ^ "The Geological Record of Ocean Acidification". JournalistsResource.org, retrieved 14 March 2012
- ^ Hönisch, Bärbel; Ridgwell, Andy; Schmidt, Daniela N.; Thomas, E.; Gibbs, S. J.; Sluijs, A.; Zeebe, R.; Kump, L.; Martindale, R. C.; Greene, S. E.; Kiessling, W.; Ries, J.; Zachos, J. C.; Royer, D. L.; Barker, S.; Marchitto, T. M.; Moyer, R.; Pelejero, C.; Ziveri, P.; Foster, G. L.; Williams, B. (2012). "The Geological Record of Ocean Acidification". Science. 335 (6072): 1058–1063. Bibcode:2012Sci...335.1058H. doi:10.1126/science.1208277. hdl:1983/24fe327a-c509-4b6a-aa9a-a22616c42d49. PMID 22383840. S2CID 6361097.
- ^ "An Ominous Warning on the Effects of Ocean Acidification by Carl Zimmer: Yale Environment 360". e360.yale.edu. Archived from the original on 16 February 2014. Retrieved 25 January 2014.
- ^ Newspapers, Les Blumenthal-McClatchy (22 April 2010). "Report: Ocean acidification rising at unprecedented rate". mcclatchydc.
- ^ United States National Research Council, 2010. Ocean Acidification: A National Strategy to Meet the Challenges of a Changing Ocean
- ^ Brander, Luke M.; Rehdanz, Katrin; Tol, Richard S. J.; Van Beukering, Pieter J. H. (1 February 2012). "The economic impact of ocean acidification on coral reefs". Climate Change Economics. 03 (1): 1250002. doi:10.1142/S2010007812500029. hdl:2262/27779. ISSN 2010-0078.
- ^ Cooley, S. R., D. J. P. Moore, S. R. Alin, D. Butman, D. W. Clow, N. H. F. French, R. A. Feely, Z. I. Johnson, G. Keppel-Aleks, S. E. Lohrenz, I. B. Ocko, E. H. Shadwick, A. J. Sutton, C. S. Potter, Y. Takatsuka, A. P. Walker, and R. M. S. Yu, 2018: Chapter 17: Biogeochemical effects of rising atmospheric carbon dioxide. In Second State of the Carbon Cycle Report (SOCCR2): A Sustained Assessment Report [Cavallaro, N., G. Shrestha, R. Birdsey, M. A. Mayes, R. G. Najjar, S. C. Reed, P. Romero-Lankao, and Z. Zhu (eds.)]. U.S. Global Change Research Program, Washington, DC, USA, pp. 690-727, https://doi.org/10.7930/SOCCR2.2018.Ch17.
- ^ Feely, R. A.; Sabine, C. L.; Hernandez-Ayon, J. M.; Ianson, D.; Hales, B. (June 2008). "Evidence for upwelling of corrosive "acidified" water onto the continental shelf". Science. 320 (5882): 1490–2. Bibcode:2008Sci...320.1490F. CiteSeerX 10.1.1.328.3181. doi:10.1126/science.1155676. PMID 18497259. S2CID 35487689. Retrieved 25 January 2014 – via Pacific Marine Environmental Laboratory (PMEL).
- ^ Feely, Richard A.; Sabine, Christopher L.; Hernandez-Ayon, J. Martin; Ianson, Debby; Hales, Burke (13 June 2008). "Evidence for Upwelling of Corrosive "Acidified" Water onto the Continental Shelf". Science. 320 (5882): 1490–1492. Bibcode:2008Sci...320.1490F. doi:10.1126/science.1155676. ISSN 0036-8075. PMID 18497259. S2CID 35487689.
- ^ Impacts of Acidification in the Mediterranean and Black Seas - Overview. 2008.pp 5-19 in CIESM Workshop Monographs n° 36 [1]
- ^ a b Key, R. M.; Kozyr, A.; Sabine, C. L.; Lee, K.; Wanninkhof, R.; Bullister, J.; Feely, R. A.; Millero, F.; Mordy, C.; Peng, T.-H. (2004). "A global ocean carbon climatology: Results from GLODAP". Global Biogeochemical Cycles. 18 (4): GB4031. Bibcode:2004GBioC..18.4031K. doi:10.1029/2004GB002247. S2CID 16428889.

- ^ "Ocean acidification and the Southern Ocean". Australian Antarctic Division — Australia in Antarctica.
- ^ "EPA weighs action on ocean acidification". 4 February 2009.
- ^ Review of Past IPCC Emissions Scenarios, IPCC Special Report on Emissions Scenarios (ISBN 0521804930).
- ^ a b Clapham, Matthew E.; Renne, Paul R. (30 May 2019). "Flood Basalts and Mass Extinctions". Annual Review of Earth and Planetary Sciences. 47 (1): 275–303. Bibcode:2019AREPS..47..275C. doi:10.1146/annurev-earth-053018-060136. ISSN 0084-6597. S2CID 133715470.
- ^ Zachos, J.C.; Röhl, U.; Schellenberg, S.A.; Sluijs, A.; Hodell, D.A.; Kelly, D.C.; Thomas, E.; Nicolo, M.; Raffi, I.; Lourens, L. J.; McCarren, H.; Kroon, D. (2005). "Rapid acidification of the ocean during the Paleocene-Eocene thermal maximum". Science. 308 (5728): 1611–1615. Bibcode:2005Sci...308.1611Z. doi:10.1126/science.1109004. hdl:1874/385806. PMID 15947184. S2CID 26909706.
- ^ Beerling, D. J.; Berner, R. A. (September 2002). "Biogeochemical constraints on the Triassic-Jurassic boundary carbon cycle event: TR-J BOUNDARY C-CYCLE DYNAMICS". Global Biogeochemical Cycles. 16 (3): 10–1–10–13. Bibcode:2002GBioC..16.1036B. doi:10.1029/2001GB001637. S2CID 53590993.
- ^ a b Hautmann, Michael; Benton, Michael J.; Tomašových, Adam (1 July 2008). "Catastrophic ocean acidification at the Triassic-Jurassic boundary". Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen. 249 (1): 119–127. doi:10.1127/0077-7749/2008/0249-0119.
- ^ Greene, Sarah E.; Martindale, Rowan C.; Ritterbush, Kathleen A.; Bottjer, David J.; Corsetti, Frank A.; Berelson, William M. (June 2012). "Recognising ocean acidification in deep time: An evaluation of the evidence for acidification across the Triassic-Jurassic boundary". Earth-Science Reviews. 113 (1–2): 72–93. Bibcode:2012ESRv..113...72G. doi:10.1016/j.earscirev.2012.03.009.
- ^ Lindström, Sofie; van de Schootbrugge, Bas; Hansen, Katrine H.; Pedersen, Gunver K.; Alsen, Peter; Thibault, Nicolas; Dybkjær, Karen; Bjerrum, Christian J.; Nielsen, Lars Henrik (July 2017). "A new correlation of Triassic–Jurassic boundary successions in NW Europe, Nevada and Peru, and the Central Atlantic Magmatic Province: A time-line for the end-Triassic mass extinction". Palaeogeography, Palaeoclimatology, Palaeoecology. 478: 80–102. Bibcode:2017PPP...478...80L. doi:10.1016/j.palaeo.2016.12.025. hdl:1874/351998. S2CID 133353132.
- ^ Hautmann, M.; Stiller, F.; Huawei, C.; Jingeng, S. (1 October 2008). "Extinction-Recovery Pattern of Level-Bottom Faunas Across the Triassic-Jurassic Boundary in Tibet: Implications for Potential Killing Mechanisms". PALAIOS. 23 (10): 711–718. Bibcode:2008Palai..23..711H. doi:10.2110/palo.2008.p08-005r. ISSN 0883-1351. S2CID 42675849.
- ^ Hautmann, Michael (15 August 2012), "Extinction: End-Triassic Mass Extinction", eLS, John Wiley & Sons, pp. a0001655.pub3, doi:10.1002/9780470015902.a0001655.pub3, ISBN 978-0-470-01617-6, S2CID 130434497
- ^ Fine, M.; Tchernov, D. (30 March 2007). "Scleractinian Coral Species Survive and Recover from Decalcification". Science. 315 (5820): 1811. Bibcode:2007Sci...315.1811F. doi:10.1126/science.1137094. ISSN 0036-8075. PMID 17395821. S2CID 28535145.
- ^ Payne, J. L.; Lehrmann, D. J.; Follett, D.; Seibel, M.; Kump, L. R.; Riccardi, A.; Altiner, D.; Sano, H.; Wei, J. (1 July 2007). "Erosional truncation of uppermost Permian shallow-marine carbonates and implications for Permian-Triassic boundary events". Geological Society of America Bulletin. 119 (7–8): 771–784. Bibcode:2007GSAB..119..771P. doi:10.1130/B26091.1. hdl:11511/35436. ISSN 0016-7606.
- ^ Clarkson, M. O.; Kasemann, S. A.; Wood, R. A.; Lenton, T. M.; Daines, S. J.; Richoz, S.; Ohnemueller, F.; Meixner, A.; Poulton, S. W.; Tipper, E. T. (10 April 2015). "Ocean acidification and the Permo-Triassic mass extinction" (PDF). Science. 348 (6231): 229–232. Bibcode:2015Sci...348..229C. doi:10.1126/science.aaa0193. ISSN 0036-8075. PMID 25859043. S2CID 28891777.
- ^ Henehan, Michael J.; Ridgwell, Andy; Thomas, Ellen; Zhang, Shuang; Alegret, Laia; Schmidt, Daniela N.; Rae, James W. B.; Witts, James D.; Landman, Neil H.; Greene, Sarah E.; Huber, Brian T. (5 November 2019). "Rapid ocean acidification and protracted Earth system recovery followed the end-Cretaceous Chicxulub impact". Proceedings of the National Academy of Sciences. 116 (45): 22500–22504. Bibcode:2019PNAS..11622500H. doi:10.1073/pnas.1905989116. ISSN 0027-8424. PMC 6842625. PMID 31636204.
- ^ Zachos, James C.; Röhl, Ursula; Schellenberg, Stephen A.; Sluijs, Appy; Hodell, David A.; Kelly, Daniel C.; Thomas, Ellen; Nicolo, Micah; Raffi, Isabella; Lourens, Lucas J.; McCarren, Heather; Kroon, Dick (10 June 2005). "Rapid Acidification of the Ocean During the Paleocene-Eocene Thermal Maximum". Science. 308 (5728): 1611–1615. Bibcode:2005Sci...308.1611Z. doi:10.1126/science.1109004. hdl:1874/385806. ISSN 0036-8075. PMID 15947184. S2CID 26909706.
- ^ a b Zeebe, R.E. (2012). "History of Seawater Carbonate Chemistry, Atmospheric CO2, and Ocean Acidification". Annual Review of Earth and Planetary Sciences. 40 (1): 141–165. Bibcode:2012AREPS..40..141Z. doi:10.1146/annurev-earth-042711-105521. S2CID 18682623.
- ^ Penman, Donald E.; Hönisch, Bärbel; Zeebe, Richard E.; Thomas, Ellen; Zachos, James C. (May 2014). "Rapid and sustained surface ocean acidification during the Paleocene-Eocene Thermal Maximum". Paleoceanography. 29 (5): 357–369. Bibcode:2014PalOc..29..357P. doi:10.1002/2014PA002621.
- ^ Gutjahr, Marcus; Ridgwell, Andy; Sexton, Philip F.; Anagnostou, Eleni; Pearson, Paul N.; Pälike, Heiko; Norris, Richard D.; Thomas, Ellen; Foster, Gavin L. (August 2017). "Very large release of mostly volcanic carbon during the Palaeocene–Eocene Thermal Maximum". Nature. 548 (7669): 573–577. Bibcode:2017Natur.548..573G. doi:10.1038/nature23646. ISSN 1476-4687. PMC 5582631. PMID 28858305.
- ^ Nina Notman (29 July 2014). "The other carbon dioxide problem". Chemistry World.
- ^ Alex Rogers (9 October 2013). "Global warming's evil twin: ocean acidification". The Conversation.
- ^ "Ocean acidification (Issues Brief)" (PDF). IUCN (International Union for Conservation of Nature). November 2017. Retrieved 3 November 2020.
- ^ a b National Research Council. Overview of Climate Changes and Illustrative Impacts. Climate Stabilization Targets: Emissions, Concentrations, and Impacts over Decades to Millennia. Washington, DC: The National Academies Press, 2011. 1. Print.
- ^ Spalding, Christopher; Finnegan, Seth; Fischer, Woodward W. (May 2017). "Energetic costs of calcification under ocean acidification". Global Biogeochemical Cycles. 31 (5): 866–877. Bibcode:2017GBioC..31..866S. doi:10.1002/2016GB005597. ISSN 0886-6236. S2CID 133783884.
- ^ Nienhuis, S.; Palmer, A.; Harley, C. (2010). "Elevated CO2 affects shell dissolution rate but not calcification rate in a marine snail". Proceedings of the Royal Society B. 277 (1693): 2553–2558. doi:10.1098/rspb.2010.0206. PMC 2894921. PMID 20392726.
- ^ a b Rosa, R.; Seibel, B. (2008). "Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator". PNAS. 105 (52): 20776–20780. Bibcode:2008PNAS..10520776R. doi:10.1073/pnas.0806886105. PMC 2634909. PMID 19075232.
- ^ a b Bibby, R.; et al. (2008). "Effects of ocean acidification on the immune response of the blue mussel Mytilus edulis". Aquatic Biology. 2: 67–74. doi:10.3354/ab00037.
- ^ "Ocean Acidification Summary for Policymakers". IGBP.
- ^ "Special Report on the Ocean and Cryosphere in a Changing Climate — Special Report on the Ocean and Cryosphere in a Changing Climate". IPCC. 25 September 2019. Retrieved 12 November 2019.
- ^ Gattuso, J.-P.; Frankignoulle, M.; Bourge, I.; Romaine, S.; Buddemeier, R. W. (1998). "Effect of calcium carbonate saturation of seawater on coral calcification". Global and Planetary Change. 18 (1–2): 37–46. Bibcode:1998GPC....18...37G. doi:10.1016/S0921-8181(98)00035-6.
- ^ Gattuso, J.-P.; Allemand, D.; Frankignoulle, M. (1999). "Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry". American Zoologist. 39: 160–183. doi:10.1093/icb/39.1.160.
- ^ Langdon, C.; Atkinson, M. J. (2005). "Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment". Journal of Geophysical Research. 110 (C09S07): C09S07. Bibcode:2005JGRC..11009S07L. doi:10.1029/2004JC002576.
- ^ Riebesell, Ulf; Zondervan, Ingrid; Rost, Björn; Tortell, Philippe D.; Zeebe, Richard E.; Morel, François M. M. (2000). "Reduced calcification of marine plankton in response to increased atmospheric CO
2" (PDF). Nature. 407 (6802): 364–367. Bibcode:2000Natur.407..364R. doi:10.1038/35030078. PMID 11014189. S2CID 4426501. - ^ Gazeau, F.; Quiblier, C.; Jansen, J. M.; Gattuso, J.-P.; Middelburg, J. J.; Heip, C. H. R. (2007). "Impact of elevated CO
2 on shellfish calcification". Geophysical Research Letters. 34 (7): L07603. Bibcode:2007GeoRL..3407603G. doi:10.1029/2006GL028554. hdl:20.500.11755/a8941c6a-6d0b-43d5-ba0d-157a7aa05668. S2CID 130190489. - ^ Buitenhuis, E. T.; de Baar, H. J. W.; Veldhuis, M. J. W. (1999). "Photosynthesis and calcification by Emiliania huxleyi (Prymnesiophyceae) as a function of inorganic carbon species". Journal of Phycology. 35 (5): 949–959. doi:10.1046/j.1529-8817.1999.3550949.x. S2CID 83502030.
- ^ Nimer, N. A.; Merrett, M. J. (1993). "Calcification rate in Emiliania huxleyi Lohmann in response to light, nitrate and availability of inorganic carbon". New Phytologist. 123 (4): 673–677. doi:10.1111/j.1469-8137.1993.tb03776.x.
- ^ a b Iglesias-Rodriguez, M.D.; Halloran, P.R.; Rickaby, R.E.M.; Hall, I.R.; Colmenero-Hidalgo, E.; Gittins, J.R.; Green, D.R.H.; Tyrrell, T.; Gibbs, S.J.; von Dassow, P.; Rehm, E.; Armbrust, E.V.; Boessenkool, K.P. (2008). "Phytoplankton Calcification in a High-CO2 World". Science. 320 (5874): 336–340. Bibcode:2008Sci...320..336I. doi:10.1126/science.1154122. PMID 18420926. S2CID 206511068.
- ^ Sciandra, A.; Harlay, J.; Lefevre, D.; et al. (2003). "Response of coccolithophorid Emiliania huxleyi to elevated partial pressure of CO2 under nitrogen limitation". Marine Ecology Progress Series. 261: 111–112. Bibcode:2003MEPS..261..111S. doi:10.3354/meps261111.
- ^ Langer, G.; Geisen, M.; Baumann, K. H.; et al. (2006). "Species-specific responses of calcifying algae to changing seawater carbonate chemistry" (PDF). Geochemistry, Geophysics, Geosystems. 7 (9): Q09006. Bibcode:2006GGG.....709006L. doi:10.1029/2005GC001227. S2CID 14774230.
- ^ "Acidification Of Oceans May Contribute To Global Declines Of Shellfish, Study By Stony Brook Scientists Concludes" (Press release). School of Marine and Atmospheric Sciences at Stony Brook University. 27 September 2010. Archived from the original on 3 September 2012. Retrieved 4 June 2012.
- ^ Turley, Carol; Gattuso, Jean-Pierre (July 2012). "Future biological and ecosystem impacts of ocean acidification and their socioeconomic-policy implications". Current Opinion in Environmental Sustainability. 4 (3): 278–286. doi:10.1016/j.cosust.2012.05.007.
- ^ Fairchild, William; Hales, Burke (2021). "High-Resolution Carbonate System Dynamics of Netarts Bay, OR From 2014 to 2019". Frontiers in Marine Science. 7. doi:10.3389/fmars.2020.590236. ISSN 2296-7745.
- ^ Ruttiman, J. (2006). "Sick Seas". Nature. 442 (7106): 978–980. Bibcode:2006Natur.442..978R. doi:10.1038/442978a. PMID 16943816. S2CID 4332965.
- ^ Cohen, A.; Holcomb, M. (2009). "Why Corals Care About Ocean Acidification: Uncovering the Mechanism". Oceanography. 24 (4): 118–127. doi:10.5670/oceanog.2009.102.
- ^ Pérez, F.; Fontela, M.; García-Ibañez, M.; Mercier, H.; Velo, A.; Lherminier, P.; Zunino, P.; de la Paz, M.; Alonso, F.; Guallart, E.; Padín, T. (22 February 2018). "Meridional overturning circulation conveys fast acidification to the deep Atlantic Ocean". Nature. 554 (7693): 515–518. Bibcode:2018Natur.554..515P. doi:10.1038/nature25493. PMID 29433125. S2CID 3497477.
- ^ Mollica, Nathaniel R.; Guo, Weifu; Cohen, Anne L.; Huang, Kuo-Fang; Foster, Gavin L.; Donald, Hannah K.; Solow, Andrew R. (20 February 2018). "Ocean acidification affects coral growth by reducing skeletal density". Proceedings of the National Academy of Sciences. 115 (8): 1754–1759. Bibcode:2018PNAS..115.1754M. doi:10.1073/pnas.1712806115. PMC 5828584. PMID 29378969.
- ^ Albright, R.; Caldeira, L.; Hosfelt, J.; Kwiatkowski, L.; Maclaren, J. K.; Mason, B. M.; Nebuchina, Y.; Ninokawa, A.; Pongratz, J.; Ricke, K. L.; Rivlin, T.; Schneider, K.; Sesboüé, M.; Shamberger, K.; Silverman, J.; Wolfe, K.; Zhu, K.; Caldeira, K. (24 February 2016). "Reversal of ocean acidification enhances net coral reef calcification". Nature. 531 (7594): 362–365. Bibcode:2016Natur.531..362A. doi:10.1038/nature17155. PMID 26909578. S2CID 205247928.
- ^ Albright, R.; Takeshita, T.; Koweek, D. A.; Ninokawa, A.; Wolfe, K.; Rivlin, T.; Nebuchina, Y.; Young, J.; Caldeira, K. (14 March 2018). "Carbon dioxide addition to coral reef waters suppresses net community calcification". Nature. 555 (7697): 516–519. Bibcode:2018Natur.555..516A. doi:10.1038/nature25968. PMID 29539634. S2CID 3935534.
- ^ Munday, Philip L. (2009). "Ocean Acidification Impairs Olfactory Discrimination and Homing Ability of a Marine Fish" (PDF). Proceedings of the National Academy of Sciences. 106 (6): 1848–52. Bibcode:2009PNAS..106.1848M. doi:10.1073/pnas.0809996106. PMC 2644126. PMID 19188596.
- ^ Hannah L. Wood; John I. Spicer; Stephen Widdicombe (2008). "Ocean acidification may increase calcification rates, but at a cost". Proceedings of the Royal Society B. 275 (1644): 1767–1773. doi:10.1098/rspb.2008.0343. PMC 2587798. PMID 18460426.
- ^ a b Ducker, James; Falkenberg, Laura J. (2020). "How the Pacific Oyster Responds to Ocean Acidification: Development and Application of a Meta-Analysis Based Adverse Outcome Pathway". Frontiers in Marine Science. 7. doi:10.3389/fmars.2020.597441. ISSN 2296-7745.
- ^ Doney, Scott C.; Busch, D. Shallin; Cooley, Sarah R.; Kroeker, Kristy J. (17 October 2020). "The Impacts of Ocean Acidification on Marine Ecosystems and Reliant Human Communities". Annual Review of Environment and Resources. 45 (1): 83–112. doi:10.1146/annurev-environ-012320-083019. ISSN 1543-5938. S2CID 225741986.
- ^ Fabricius, Katharina (2011). "Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations". Nature Climate Change. 1 (3): 165–169. Bibcode:2011NatCC...1..165F. doi:10.1038/nclimate1122. S2CID 85749253.
- ^ Henehan, Michael J.; Ridgwell, Andy; Thomas, Ellen; Zhang, Shuang; Alegret, Laia; Schmidt, Daniela N.; Rae, James W. B.; Witts, James D.; Landman, Neil H.; Greene, Sarah E.; Huber, Brian T. (5 November 2019). "Rapid ocean acidification and protracted Earth system recovery followed the end-Cretaceous Chicxulub impact". Proceedings of the National Academy of Sciences. 116 (45): 22500–22504. Bibcode:2019PNAS..11622500H. doi:10.1073/pnas.1905989116. ISSN 0027-8424. PMC 6842625. PMID 31636204.
- ^ Petrou, Katherina; Nielsen, Daniel (27 August 2019). "Acid oceans are shrinking plankton, fueling faster climate change". phys.org. Retrieved 12 November 2019.
- ^ Hoegh-Guldberg, Ove; Poloczanska, Elvira S.; Skirving, William; Dove, Sophie (2017). "Coral Reef Ecosystems under Climate Change and Ocean Acidification". Frontiers in Marine Science. 4: 158. doi:10.3389/fmars.2017.00158. ISSN 2296-7745.
- ^ Cooley, S., D. Schoeman, L. Bopp, P. Boyd, S. Donner, D.Y. Ghebrehiwet, S.-I. Ito, W. Kiessling, P. Martinetto, E. Ojea, M.-F. Racault, B. Rost, and M. Skern-Mauritzen, 2022: Chapter 3: Oceans and Coastal Ecosystems and Their Services. In: Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [H.-O. Pörtner, D.C. Roberts, M. Tignor, E.S. Poloczanska, K. Mintenbeck, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller, A. Okem, B. Rama (eds.)]. Cambridge University Press, Cambridge, UK and New York, NY, USA, pp. 379–550, doi:10.1017/9781009325844.005.
- ^ Hester, K. C.; et al. (2008). "Unanticipated consequences of ocean acidification: A noisier ocean at lower pH". Geophysical Research Letters. 35 (19): L19601. Bibcode:2008GeoRL..3519601H. doi:10.1029/2008GL034913.
- ^ Acid In The Oceans: A Growing Threat To Sea Life by Richard Harris. All Things Considered, 12 August 2009.
- ^ Kwok, Roberta (4 June 2013). "Ocean acidification could make squid develop abnormally". University of Washington. Retrieved 24 August 2013.
- ^ "Swiss marine researcher moving in for the krill". The Australian. 2008. Archived from the original on 11 December 2008. Retrieved 28 September 2008.
- ^ "Ocean Acidification Promotes Disruptive and Harmful Algal Blooms on Our Coasts". 2014.
- ^ a b Turley, Carol; Gattuso, Jean-Pierre (July 2012). "Future biological and ecosystem impacts of ocean acidification and their socioeconomic-policy implications". Current Opinion in Environmental Sustainability. 4 (3): 278–286. doi:10.1016/j.cosust.2012.05.007.
- ^ a b c Munday, Philip L. (2009). "Ocean Acidification Impairs Olfactory Discrimination and Homing Ability of a Marine Fish" (PDF). Proceedings of the National Academy of Sciences. 106 (6): 1848–52. Bibcode:2009PNAS..106.1848M. doi:10.1073/pnas.0809996106. PMC 2644126. PMID 19188596.
- ^ Clements, Jeff C.; Sundin, Josefin; Clark, Timothy D.; Jutfelt, Fredrik (3 February 2022). "Meta-analysis reveals an extreme "decline effect" in the impacts of ocean acidification on fish behavior". PLOS Biology. 20 (2): e3001511. doi:10.1371/journal.pbio.3001511. ISSN 1545-7885. PMC 8812914. PMID 35113875.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Chan, F., Barth, J.A., Kroeker, K.J., Lubchenco, J. and Menge, B.A. (2019) "The dynamics and impact of ocean acidification and hypoxia". Oceanography, 32(3): 62–71. doi:10.5670/oceanog.2019.312.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Gewin, V. (2010) "Oceanography: Dead in the water". Nature, 466(7308): 812. doi:10.1038/466812a.
- ^ Kroeker, et al. (June 2013) "Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming." Glob Chang Biol. 19(6): 1884–1896
- ^ Harvey, et al. (April 2013) "Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming." Ecol Evol. 3(4): 1016–1030
- ^ Nagelkerken Global alteration of ocean ecosystem functioning due to increasing human CO2 emissions, PNAS vol. 112 no. 43, 2015
- ^ Bednaršek, N.; Harvey, C.J.; Kaplan, I.C.; Feely, R.A.; Možina, J. (2016). "Pteropods on the edge: Cumulative effects of ocean acidification, warming, and deoxygenation". Progress in Oceanography. 145: 1–24. Bibcode:2016PrOce.145....1B. doi:10.1016/j.pocean.2016.04.002.
- ^ Keeling, Ralph F.; Garcia, Hernan E. (2002). "The change in oceanic O2 inventory associated with recent global warming". Proceedings of the National Academy of Sciences. 99 (12): 7848–7853. Bibcode:2002PNAS...99.7848K. doi:10.1073/pnas.122154899. PMC 122983. PMID 12048249.
- ^ Harvey wt al Ecol Evol. 2013 Apr; 3(4): 1016–1030
- ^ Gruber, Nicolas. "Warming up, turning sour, losing breath: ocean biogeochemistry under global change." Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences 369.1943 (2011): 1980–1996.
- ^ Anthony, et al. (May 2011) "Ocean acidification and warming will lower coral reef resilience." Global Change biology, Volume 17, Issue 5, Pages 1798–1808
- ^ Goldenberg, Silvan U, et al. (2017) "Boosted food web productivity through ocean acidification collapses under warming." Global Change Biology.
- ^ Pistevos, Jennifer CA, et al. (2015) "Ocean acidification and global warming impair shark hunting behaviour and growth." Scientific reports 5: 16293.
- ^ "Coral reefs". WWF. Retrieved 6 May 2019.
- ^ Fabry, Victoria J.; Seibel, Brad A.; Feely, Richard A.; Orr, James C. (April 2008). "Impacts of ocean acidification on marine fauna and ecosystem processes". ICES Journal of Marine Science. 65 (3): 414–432. doi:10.1093/icesjms/fsn048.
- ^ Doney, Scott C.; Busch, D. Shallin; Cooley, Sarah R.; Kroeker, Kristy J. (17 October 2020). "The Impacts of Ocean Acidification on Marine Ecosystems and Reliant Human Communities". Annual Review of Environment and Resources. 45 (1): 83–112. doi:10.1146/annurev-environ-012320-083019. ISSN 1543-5938. S2CID 225741986.
- ^ Hoegh-Guldberg, O.; Mumby, P. J.; Hooten, A. J.; Steneck, R. S.; Greenfield, P.; Gomez, E.; Harvell, C. D.; Sale, P. F.; Edwards, A. J.; Caldeira, K.; Knowlton, N.; Eakin, C. M.; Iglesias-Prieto, R.; Muthiga, N.; Bradbury, R. H.; Dubi, A.; Hatziolos, M. E. (14 December 2007). "Coral Reefs Under Rapid Climate Change and Ocean Acidification". Science. 318 (5857): 1737–1742. Bibcode:2007Sci...318.1737H. doi:10.1126/science.1152509. hdl:1885/28834. PMID 18079392. S2CID 12607336.
- ^ "Effects of Ocean Acidification on Marine Species & Ecosystems". Report. OCEANA. Retrieved 13 October 2013.
- ^ a b Lischka, S.; Büdenbender J.; Boxhammer T.; Riebesell U. (15 April 2011). "Impact of ocean acidification and elevated temperatures on early juveniles of the polar shelled pteropod Limacina helicina : mortality, shell degradation, and shell growth" (PDF). Report. Biogeosciences. pp. 919–932. Retrieved 14 November 2013.
- ^ "Comprehensive study of Arctic Ocean acidification". Study. CICERO. Archived from the original on 10 December 2013. Retrieved 14 November 2013.
- ^ "Antarctic marine wildlife is under threat, study finds". BBC Nature. Retrieved 13 October 2013.
- ^ V. J. Fabry; C. Langdon; W. M. Balch; A. G. Dickson; R. A. Feely; B. Hales; D. A. Hutchins; J. A. Kleypas & C. L. Sabine. "Present and Future Impacts of Ocean Acidification on Marine Ecosystems and Biogeochemical Cycles" (PDF). Report of the Ocean Carbon and Biogeochemistry Scoping Workshop on Ocean Acidification Research. Archived from the original (PDF) on 17 November 2010. Retrieved 14 November 2013.
- ^ "Canada's State of the Oceans Report, 2012". Report. Fisheries and Oceans Canada. 2012. Archived from the original on 6 November 2013. Retrieved 21 October 2013.
- ^ Robert J. Foy; Mark Carls; Michael Dalton; Tom Hurst; W. Christopher Long; Dusanka Poljak; André E. Punt; Michael F. Sigler; Robert P. Stone; Katherine M. Swiney (Winter 2013). "CO 2 , pH, and Anticipating a Future under Ocean Acidification" (PDF). ONCORHYNCHUS. Vol. XXXIII, no. 1. Retrieved 14 November 2013.
- ^ "Bering Sea Crab Fishery". Report. Seafood Market Bulletin. November 2005. Archived from the original on 11 December 2013. Retrieved 10 November 2013.
- ^ Lynn, Kathy; Daigle, John; Hoffman, Jennie; Lake, Frank; Michelle, Natalie; Ranco, Darren; Viles, Carson; Voggesser, Garrit; Williams, Paul (2014), Maldonado, Julie Koppel; Colombi, Benedict; Pandya, Rajul (eds.), "The impacts of climate change on tribal traditional foods", Climate Change and Indigenous Peoples in the United States: Impacts, Experiences and Actions, Cham: Springer International Publishing, pp. 37–48, doi:10.1007/978-3-319-05266-3_4, ISBN 978-3-319-05266-3, retrieved 30 October 2022
- ^ a b Boudreau, Bernard P.; Middelburg, Jack J.; Hofmann, Andreas F.; Meysman, Filip J. R. (2010). "Ongoing transients in carbonate compensation: COMPENSATION TRANSIENTS". Global Biogeochemical Cycles. 24 (4): n/a. doi:10.1029/2009GB003654. S2CID 53062358.
- ^ Sulpis, Olivier; et al. (29 October 2018). "Current CaCO3 dissolution at the seafloor caused by anthropogenic CO2". Proceedings of the National Academy of Sciences of the United States of America. 115 (46): 11700–11705. Bibcode:2018PNAS..11511700S. doi:10.1073/pnas.1804250115. PMC 6243283. PMID 30373837.
- ^ Ridgwell, A.; Zondervan, I.; Hargreaves, J. C.; Bijma, J.; Lenton, T. M. (2007). "Assessing the potential long-term increase of oceanic fossil fuel CO2 uptake due to CO2-calcification feedback". Biogeosciences. 4 (4): 481–492. Bibcode:2007BGeo....4..481R. doi:10.5194/bg-4-481-2007.
- ^ Tyrrell, T. (2008). "Calcium carbonate cycling in future oceans and its influence on future climates". Journal of Plankton Research. 30 (2): 141–156. doi:10.1093/plankt/fbm105.
- ^ Harvey, Fiona (4 December 2019). "Tackling degraded oceans could mitigate climate crisis - report". The Guardian. ISSN 0261-3077. Retrieved 7 December 2019.
- ^ "How Can Trees Help the Seas?". The Nature Conservancy. Retrieved 3 September 2021.
- ^ a b c d e f g National Academies of Sciences, Engineering, and Medicine. 2022. A Research Strategy for Ocean-based Carbon Dioxide Removal and Sequestration. Washington, DC: The National Academies Press. https://doi.org/10.17226/26278.
- ^ Matear, R. J. & B. Elliott (2004). "Enhancement of oceanic uptake of anthropogenic CO2 by macronutrient fertilization". J. Geophys. Res. 109 (C4): C04001. Bibcode:2004JGRC..109.4001M. doi:10.1029/2000JC000321. Archived from the original on 4 March 2010. Retrieved 19 January 2009.
- ^ Jones, I.S.F. & Young, H.E. (1997). "Engineering a large sustainable world fishery". Environmental Conservation. 24 (2): 99–104. Bibcode:1997EnvCo..24...99J. doi:10.1017/S0376892997000167. S2CID 86248266.
- ^ Trujillo, Alan (2011). Essentials of Oceanography. Pearson Education, Inc. p. 157. ISBN 9780321668127.
- ^ a b National Academies of Sciences, Engineering (8 December 2021). A Research Strategy for Ocean-based Carbon Dioxide Removal and Sequestration. doi:10.17226/26278. ISBN 978-0-309-08761-2. PMID 35533244. S2CID 245089649.
- ^ "Action Plans". OA Alliance. Retrieved 4 November 2022.
- ^ "Cartagena Convention | The Caribbean Environment Programme (CEP)". www.unep.org. Retrieved 4 November 2022.
- ^ Turner, J; McIntosh, R. Duncan (2019). Mainstreaming Ocean Acidification into National Policies: A Handbook for Pacific Islands (PDF). Apia, Samoa: Secretariat of the Pacific Regional Environment Programme.
- ^ "The Ocean Decade - The Science we need for the Ocean we want". Ocean Decade. Retrieved 4 November 2022.
- ^ "GOA-ON : OARS". goa-on.org. Retrieved 4 November 2022.
- ^ "GCOS | WMO". gcos.wmo.int. Retrieved 4 November 2022.
- ^ "Home | GCOS". gcos.wmo.int. Retrieved 4 November 2022.
- ^ United Nations (2017) Resolution adopted by the General Assembly on 6 July 2017, Work of the Statistical Commission pertaining to the 2030 Agenda for Sustainable Development (A/RES/71/313)
- ^ "Goal 14: Sustainable Development Knowledge Platform". sustainabledevelopment.un.org. Retrieved 5 September 2020.
- ^ "Update on IOC Custodianship Role in Relation to SDG 14 Indicators". unesdoc.unesco.org. Retrieved 4 November 2022.
- ^ "SDG 14.3.1 data portal". oa.iode.org. Retrieved 4 November 2022.
- ^ Galdies, Charles; Bellerby, Richard; Canu, Donata; Chen, Wenting; Garcia-Luque, Enrique; Gašparović, Blaženka; Godrijan, Jelena; Lawlor, Paul J.; Maes, Frank; Malej, Alenka; Panagiotaras, Dionisios; Romera, Beatriz Martinez; Reymond, Claire E.; Rochette, Julien; Solidoro, Cosimo (1 August 2020). "European policies and legislation targeting ocean acidification in european waters - Current state". Marine Policy. 118: 103947. doi:10.1016/j.marpol.2020.103947. ISSN 0308-597X. S2CID 218961473.
- ^ "OAP Home". oceanacidification.noaa.gov. Retrieved 4 November 2022.
- ^ Center for Biological Diversity; Donn J. Viviani. "TSCA Section 21 Petition Requesting EPA to Regulate Anthropogenic Emissions Carbon Dioxide" (PDF). US EPA.
- ^ "Carbon Dioxide Emissions and Ocean Acidification; TSCA Section 21 Petition; Reasons for Agency Response". Environmental Protection Agency (EPA). 7 October 2015.
- ^ "The President's Climate Action Plan" (PDF). Retrieved 27 June 2017.

![{\displaystyle {\Omega }={\frac {\left[{\ce {Ca^2+}}\right]\left[{\ce {CO3^2-}}\right]}{K_{sp}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0422ec8bbb280ae8547a20d486b928b0c21846f4)