Search results
Appearance
There is a page named "Reduction-oxidation" on Wikipedia
- Redox (redirect from Oxidation-reduction reaction)reduction–oxidation or oxidation–reduction: 150 ) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is...37 KB (3,583 words) - 17:38, 9 July 2024
- Organic redox reaction (redirect from Organic oxidation)its oxidation number changes from −4 to +4. Classical reductions include alkene reduction to alkanes and classical oxidations include oxidation of alcohols...6 KB (672 words) - 19:09, 3 March 2024
- Redox potential (also known as oxidation / reduction potential, ORP, pe, E r e d {\displaystyle E_{red}} , or E h {\displaystyle E_{h}} ) is a measure...24 KB (3,173 words) - 23:59, 24 February 2024
- Reducing agent (redirect from Reduction agent)terms of their oxidation states. An agent's oxidation state describes its degree of loss of electrons, where the higher the oxidation state then the fewer...15 KB (1,903 words) - 19:02, 8 July 2024
- Oxidizing agent (redirect from Oxidation half reaction)expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents...9 KB (875 words) - 10:33, 12 May 2024
- Electrochemistry (redirect from Electrochemical reduction)for reduction-oxidation. It refers to electrochemical processes involving electron transfer to or from a molecule or ion, changing its oxidation state...62 KB (8,003 words) - 01:46, 7 July 2024
- In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. It...45 KB (14,066 words) - 11:21, 1 July 2024
- or reduction in Wiktionary, the free dictionary. Reduction, reduced, or reduce may refer to: Reduction (chemistry), part of a reduction-oxidation (redox)...8 KB (1,078 words) - 01:25, 2 November 2023
- Raku ware (section Reduction process)Arbuckle. "Reduction Firing." Reduction Firing. Web. 6 May 2010. <http://lindaarbuckle.com/handouts/reduction_fire.pdf>. "Oxidation/Reduction Firing." Frog...26 KB (3,459 words) - 06:09, 3 July 2024
- Sulfur cycle (redirect from Thermochemical sulfate reduction)well as sulfide minerals. Oxidation of hydrogen sulfide, sulfide, and elemental sulfur (S) to sulfate (SO2− 4). Reduction of sulfate to sulfide. Incorporation...59 KB (7,162 words) - 10:24, 19 May 2024
- entail partners that differ by more than their oxidation states. One example (of many thousands) is the reduction of permanganate by iodide to form iodine and...6 KB (805 words) - 12:08, 3 May 2024
- Redox (reduction–oxidation reaction) is a chemical reaction in which the oxidation states of atoms are changed. Redox may also refer to: Redox (operating...445 bytes (90 words) - 12:08, 12 November 2019
- The Oppenauer oxidation is still used for the oxidation of acid labile substrates. The method has been largely displaced by oxidation methods based on...12 KB (1,250 words) - 17:28, 13 May 2022
- Calvin cycle (redirect from Reductive pentose phosphate pathway)sugars for the plant to use. These substrates are used in a series of reduction-oxidation (redox) reactions to produce sugars in a step-wise process; there...23 KB (2,704 words) - 11:41, 13 June 2024
- metal powder and metal oxide. When ignited by heat or chemical reaction, thermite undergoes an exothermic reduction-oxidation (redox) reaction. Most varieties...49 KB (5,765 words) - 13:14, 22 June 2024
- which the oxidation reaction occurs. In a galvanic cell the anode is the wire or plate having excess negative charge as a result of the oxidation reaction...20 KB (2,569 words) - 21:11, 27 May 2024
- the reduction reaction to the right according to Le Chatelier's principle. For the oxidation of reducing agents, the reverse occurs: as the oxidation state...31 KB (3,494 words) - 14:28, 14 June 2024
- developed. Oppenauer oxidation Carbonyl reduction Wilds, A. L. (1944). "Reduction with Aluminum Alkoxides (The Meerwein-Ponndorf-Verley Reduction)". Organic Reactions...14 KB (1,512 words) - 00:33, 18 April 2024
- Nernst equation (redirect from Formal standard reduction potential)approximated by concentrations) of the chemical species undergoing reduction and oxidation respectively. It was named after Walther Nernst, a German physical...47 KB (6,926 words) - 03:26, 11 June 2024
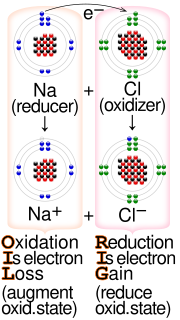
- oxidation-reduction (not comparable) Synonym of redox
- oxidizing agent suffers reduction and the reducing agent oxidation. Many substances undergo simultaneous oxidation and reduction when treated in a particular
- Redox (portmanteau of reduction and oxidation) reactions include all chemical reactions in which atoms have their oxidation state changed; in general
- Oxidation refers to the loss of electrons, while reduction refers to the gain of electrons. A full reaction will have a net change of 0 electrons, meaning