Search results
Appearance
There is a page named "P-Toluenesulfonyl chloride" on Wikipedia
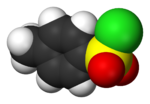
- 4-Toluenesulfonyl chloride (p-toluenesulfonyl chloride, toluene-p-sulfonyl chloride) is an organic compound with the formula CH3C6H4SO2Cl. This white...6 KB (422 words) - 16:14, 28 July 2024
- Tosyl azide (redirect from P-Toluenesulfonyl azide)Heydt, H.; Regitz, M.; Mapp, A. K.; Chen, B. (2008). "P-Toluenesulfonyl Azide". p -Toluenesulfonyl Azide. Encyclopedia of Reagents for Organic Synthesis...2 KB (171 words) - 14:21, 28 February 2024
- contrast to the formation of toluenesulfonates from alcohols and p-toluenesulfonyl chloride in the presence of pyridine, the formation of methanesulfonates...9 KB (772 words) - 16:07, 17 May 2024
- a reagent in organic synthesis. Toluenesulfonyl hydrazide is prepared by the reaction of a toluenesulfonyl chloride with hydrazine: Tosylhydrazides can...4 KB (325 words) - 18:51, 9 August 2024
- Tosyl group (redirect from P-toluenesulfonyl)In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula −SO2−C6H4−CH3...7 KB (781 words) - 19:17, 13 April 2024
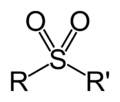
- Sulfonyl halide (redirect from Sulfonyl chloride)sulfonamide. Using sodium sulfite as the nucleophilic reagent, p-toluenesulfonyl chloride is converted to its sulfinate salt, CH3C6H4SO2Na. Chlorosulfonated...12 KB (1,352 words) - 19:02, 13 March 2024
- p-toluenesulfonate (from dimethylformamide and p-toluenesulfonyl chloride) were investigated. N,N,N′,N′-Tetramethylformamidinium chloride is useful...13 KB (1,125 words) - 19:55, 8 May 2023
- carbodiimides: (R(H)N)2CO → (RN)2C + H2O Phosphorus pentoxide and p-Toluenesulfonyl chloride have been used as a dehydrating agents. Isocyanates can be converted...14 KB (1,599 words) - 12:14, 21 July 2024
- sulfonic acids such as trifluoromethanesulfonic anhydride or p-toluenesulfonyl chloride are used as so-called photoacids, which split off protons during...19 KB (1,927 words) - 16:55, 7 March 2024
- anti-7-norbornyl p-toluenesulfonate. Tosylates are also protecting groups for alcohols. They are prepared by combining the alcohol with 4-toluenesulfonyl chloride in...9 KB (635 words) - 02:51, 31 July 2024
- 11α-hydroxyl of the starting material (1.16) is sulfonylated by p-toluenesulfonyl chloride; addition of trimethylamine (base) deprotonates the 11α-carbon...27 KB (2,325 words) - 08:10, 2 February 2024
- Example Tosyl p-toluenesulfonyl Ts Tosyl chloride (p-toluenesulfonyl chloride) CH3C6H4SO2Cl Brosyl p-bromobenzenesulfonyl Bs Nosyl o- or p-nitrobenzenesulfonyl...2 KB (201 words) - 20:23, 29 April 2023
- amines and alcohols, respectively. The closely related compound toluenesulfonyl chloride is often preferred analogue because it is a solid at room temperature...2 KB (172 words) - 19:43, 27 October 2022
- the reaction of toluene with ClSO2OH to give the ortho- and para-toluenesulfonyl chloride derivatives: CH3C6H5 + 2 ClSO2OH → CH3C6H4SO2Cl + H2SO4 + HCl Oxidation...8 KB (515 words) - 22:28, 21 May 2024
- hydroxyl group in 36 was converted to a good leaving group using p-toluenesulfonyl chloride. Subsequent deprotection of the trimethylsilyl ether 37 gave tosylate...16 KB (1,478 words) - 18:34, 27 September 2023
- dehydration of formamides. The formamide can be dehydrated with toluenesulfonyl chloride, phosphorus oxychloride, phosgene, diphosgene, or the Burgess reagent...17 KB (1,913 words) - 20:09, 4 February 2024
- chloroformate, 1,2-dichloroethane, reflux; (2) MeOH reflux; (b) p-toluenesulfonyl chloride, triethylamine; (c) LiAlH4, THF, rt; (d) trifluoromethanesulfonic...6 KB (477 words) - 05:59, 21 June 2022
- tosyl (tosylate) esters are made by reaction of the alcohol with 4-toluenesulfonyl chloride in pyridine. Primary alcohols (R−CH2OH) can be oxidized either...34 KB (3,818 words) - 08:17, 6 August 2024
- Hydrazine (redirect from Hydrazinium chloride)15227/orgsyn.031.0043. Friedman L, Litle RL, Reichle WR (1960). "p-Toluenesulfonyl Hydrazide". Org. Synth. 40: 93. doi:10.15227/orgsyn.040.0093. Weinshenker...51 KB (5,273 words) - 06:02, 29 July 2024
- demonstrated that with a mixture of hydrogen iodide and phosphonium iodide, p-toluenesulfonyl amino acids could be detosylated reductively. Schoenheimer used the...25 KB (3,025 words) - 11:23, 11 February 2024
- hydrazocarbonamide, benzenesulfonyl hydrazide, dinitrosopentamethylene tetramine, toluenesulfonyl hydrazide, benzene sulfonyl hydrazide, azobisisobutyronitrile and barium
- (thionyl chloride) is used for converting alcohols to alkyl chlorides. THF (tetrahydrofuran) is used for medium polarity solvent. pTsCl [p-toluenesulfonyl chloride