Search results
Appearance
There is a page named "Nucleotide sugar" on Wikipedia
- Nucleotide sugars are the activated forms of monosaccharides. Nucleotide sugars act as glycosyl donors in glycosylation reactions. Those reactions are...8 KB (903 words) - 13:38, 24 October 2024
- Nucleotides are organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers...32 KB (3,305 words) - 04:15, 28 January 2025
- In nucleotide sugar metabolism a group of biochemicals known as nucleotide sugars act as donors for sugar residues in the glycosylation reactions that...4 KB (463 words) - 02:49, 9 November 2021
- They catalyze the transfer of saccharide moieties from an activated nucleotide sugar (also known as the "glycosyl donor") to a nucleophilic glycosyl acceptor...14 KB (1,566 words) - 05:14, 19 September 2024
- Nicotinamide adenine dinucleotide (redirect from Diphosphopyridine nucleotide)a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other...81 KB (9,219 words) - 18:15, 16 March 2025
- Synthesis originates from glucose as substrate for synthesis of the sugar nucleotides precursors UDP-glucose, UDP-glucuronate, and GDP-mannose that are...15 KB (1,721 words) - 16:26, 23 March 2025
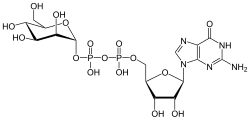
- Uridine diphosphate glucose (category Nucleotides)UDP-glucose) is a nucleotide sugar. It is involved in glycosyltransferase reactions in metabolism. UDP-glucose is used in nucleotide sugar metabolism as an...3 KB (135 words) - 00:46, 8 May 2023
- monomers called nucleotides. Nucleotide synthesis is an anabolic mechanism generally involving the chemical reaction of phosphate, pentose sugar, and a nitrogenous...14 KB (1,457 words) - 14:51, 9 January 2025
- Nucleoside triphosphate (redirect from Nucleotide triphosphates)refers to a nitrogenous base linked to a 5-carbon sugar (either ribose or deoxyribose). Nucleotides are nucleosides covalently linked to one or more phosphate...24 KB (2,518 words) - 06:30, 20 January 2025
- the two sugar-rings of two adjacent nucleotide monomers, thereby creating a long chain biomolecule. These chain-joins of phosphates with sugars (ribose...15 KB (1,439 words) - 13:26, 2 February 2025
- cyclic nucleotide (cNMP) is a single-phosphate nucleotide with a cyclic bond arrangement between the sugar and phosphate groups. Like other nucleotides, cyclic...21 KB (2,416 words) - 20:13, 5 December 2024
- other pathway is conversion of a ribose sugar into a deoxyribose sugar by means of changes on the nucleotide or nucleoside level. Deoxyribose is synthesized...3 KB (247 words) - 04:39, 21 February 2023
- cell membrane. Glycogenolysis is regulated hormonally in response to blood sugar levels by glucagon and insulin, and stimulated by epinephrine during the...6 KB (673 words) - 06:10, 27 January 2025
- Guanosine diphosphate mannose (category Nucleotides)Guanosine diphosphate mannose or GDP-mannose is a nucleotide sugar that is a substrate for glycosyltransferase reactions in metabolism. This compound is...4 KB (231 words) - 12:14, 21 October 2024
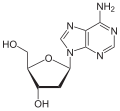
- of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar (ribose...10 KB (813 words) - 13:31, 2 February 2025
- (SLC33A1) type II Na+-phosphate cotransporter (SLC34A1, SLC34A2, SLC34A3) nucleotide-sugar transporter subfamily A (SLC35A1, SLC35A2, SLC35A3, SLC35A4, SLC35A5)...35 KB (3,408 words) - 02:42, 30 November 2023
- Nucleoside analogue (redirect from Nucleotide analog)nucleoside, which normally contain a nucleobase and a sugar. Nucleotide analogues are analogues of a nucleotide, which normally has one to three phosphates linked...7 KB (717 words) - 17:44, 16 June 2024
- the proton motive force. The ATP-ADP translocase (also called adenine nucleotide translocase, ANT) is an antiporter and exchanges ADP and ATP across the...31 KB (3,374 words) - 00:32, 22 March 2025
- purification from yeast extracts, this heat-stable factor was identified as a nucleotide sugar phosphate by Hans von Euler-Chelpin. Other cofactors were identified...48 KB (4,938 words) - 05:43, 4 February 2025
- Metabolism (section Nucleotide synthesis and salvage)and RNA, are polymers of nucleotides. Each nucleotide is composed of a phosphate attached to a ribose or deoxyribose sugar group which is attached to...114 KB (12,436 words) - 22:20, 4 March 2025
- and nucleotides in an RNA world in the first place, that it would be a dirty RNA world contaminated with fatty acids and amino acids, and sugars and things
- Resistant plants have evolved to recognize effectors via intracellular nucleotide binding site (NBS) and the leucine-rich (LRR) repeat domain or resistance
- deoxyribose sugar. A nucleoside can form a glycosidic bond linking to 1+ phophate group. A nucleoside + one phosphate makes a nucleotide. Nucleic acid