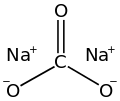
Search results
Appearance
There is a page named "NaAsO2" on Wikipedia
- Sodium arsenite (redirect from NaAsO2)NaAsO2. Also called sodium meta-arsenite, it is an inorganic polymer consisting of the infinite chains [AsO2]n− n associated with sodium cations, Na+...6 KB (543 words) - 00:34, 2 July 2024
- Sodium aluminate (redirect from NaAlO2)(anhydrous) is a white crystalline solid having a formula variously given as NaAlO2, NaAl(OH)4 (hydrated), Na2O·Al2O3, or Na2Al2O4. Commercial sodium aluminate...7 KB (648 words) - 09:38, 11 April 2024
- Sodium benzoate (redirect from C7H5NaO2)and heat, yielding benzene: C 6 H 5 COONa + NaOH ⟶ C 6 H 6 + Na 2 CO 3 {\displaystyle {\ce {C6H5COONa + NaOH -> C6H6 + Na2CO3}}} Many foods are natural...22 KB (1,823 words) - 12:13, 30 July 2024
- Sodium acetate (redirect from C2H3NaO2)Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses. Sodium...18 KB (1,281 words) - 15:04, 20 August 2024
- Sodium chlorite (redirect from NaClO2)Sodium chlorite (NaClO2) is a chemical compound used in the manufacturing of paper and as a disinfectant. The main application of sodium chlorite is the...22 KB (1,815 words) - 00:11, 3 May 2024
- Sodium-ion battery (redirect from Na-ion battery)iron-based materials (such as NaFeO2 with the Fe3+/Fe4+ redox pair) work well in Na+ batteries. This is because the ionic radius of Na+ (116 pm) is substantially...66 KB (6,810 words) - 13:29, 5 August 2024
- Sodium cyanide (redirect from NaCN)sodium dicyanoaurate (or sodium gold cyanide) (NaAu(CN)2): 4 Au + 8 NaCN + O2 + 2 H2O → 4 Na[Au(CN)2] + 4 NaOH A similar process uses potassium cyanide (KCN...9 KB (765 words) - 11:07, 24 May 2024
- Sodium hydroxide (redirect from Na(OH))is a white crystalline solid having a formula variously given as NaAlO2, Na3AlO3, Na[Al(OH)4], Na2O·Al2O3 or Na2Al2O4. Formation of sodium tetrahydroxoaluminate(III)...54 KB (5,892 words) - 18:16, 8 August 2024
- Sodium superoxide (redirect from NaO2)the formula NaO2. This yellow-orange solid is a salt of the superoxide anion. It is an intermediate in the oxidation of sodium by oxygen. NaO2 is prepared...4 KB (205 words) - 07:36, 30 January 2024
- Sodium bicarbonate (redirect from NaHCO3)of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation (Na+) and a bicarbonate anion (HCO3−). Sodium bicarbonate...56 KB (5,316 words) - 05:19, 23 July 2024
- sodium compounds like borax. Sodium carbonate serves as a flux for silica (SiO2, melting point 1,713 °C), lowering the melting point of the mixture to something...32 KB (3,138 words) - 14:54, 19 August 2024
- method: 2 NaClO2 + 2 HCl + NaOCl → 2 ClO2 + 3 NaCl + H2O or the sodium chlorite–hydrochloric acid method: 5 NaClO2 + 4 HCl → 5 NaCl + 4 ClO2 + 2 H2O or...40 KB (3,913 words) - 10:06, 7 June 2024
- Sodium chloride (redirect from Na Cl)commonly known as edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chlorine ions. It is transparent...32 KB (3,459 words) - 12:15, 7 August 2024
- Sodium hypochlorite (redirect from NaOCl)O2 In hot sodium hypochlorite solutions, this reaction competes with chlorate formation, yielding sodium chloride and oxygen gas: 2 NaOCl(aq) → 2 NaCl(aq)...55 KB (5,763 words) - 14:01, 16 August 2024
- formula Na 2xSi yO 2y+x or (Na 2O) x·(SiO 2) y, such as sodium metasilicate Na 2SiO 3, sodium orthosilicate Na 4SiO 4, and sodium pyrosilicate Na 6Si 2O...38 KB (4,697 words) - 21:03, 15 August 2024
- processes is known as salt cake. Mannheim: 2 NaCl + H2SO4 → 2 HCl + Na2SO4 Hargreaves: 4 NaCl + 2 SO2 + O2 + 2 H2O → 4 HCl + 2 Na2SO4 The second major...28 KB (2,848 words) - 18:08, 3 April 2024
- respectively. Upon dissolution in water, bisulfite is generated: Na2S2O5 + H2O → 2 Na+ + 2 HSO3− Sodium and potassium metabisulfite have many major and niche uses...13 KB (1,172 words) - 16:26, 19 August 2024
- odorless, crystalline powder. The solid contains separate sodium cations Na+ and glutamate anions in zwitterionic form, −OOC-CH(NH+ 3)-(CH 2)2-COO−. In...42 KB (4,414 words) - 18:10, 17 August 2024
- of thioglycolic acid: ClCH2CO2H + Na2S2O3 → Na[O3S2CH2CO2H] + NaCl Na[O3S2CH2CO2H] + H2O → HSCH2CO2H + NaHSO4 Sodium thiosulfate has low toxicity. LDLo...14 KB (1,298 words) - 01:19, 18 July 2024
- higher acidic oxide. Examples are BaO2+H2SO4=BaSO4+H2O2.; 2MnO2+2H2SO4=2MnSO4-2H2O+O2; MnO2+4HCl=MnCl, +2H2O + Cl2; PbO2+SO2=PbSO4 (i.e. PbO+SO3). Two species
- esters by MnO2 and NaCN in an alcohol solvent. Sodium cyanide catalyzes the oxidation by forming a cyanohydrin that is susceptible to MnO2 oxidation. Methanolysis
- NaNO3 → NaNO2 + O2 9. Cu(NO3)2 → CuO + NO2 + O2 10. PbO + C → Pb + CO2 11. C4H8 + O2 → CO2 + H2O 12. C4H10 + O2 → CO2 + H2O 13. Na2CO3 + HCl → NaCl