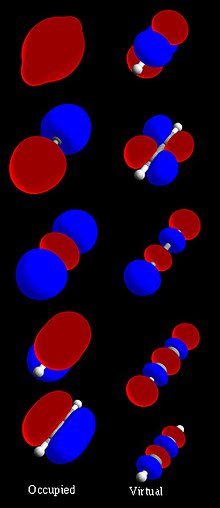
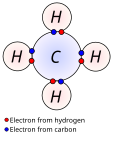
Search results
Appearance
There is a page named "Molecular electron transition" on Wikipedia
- In theoretical chemistry, molecular electronic transitions take place when electrons in a molecule are excited from one energy level to a higher energy...5 KB (533 words) - 03:04, 24 August 2023
- chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals...60 KB (6,147 words) - 22:00, 18 May 2024
- and chemistry, an atomic electron transition (also called an atomic transition, quantum jump, or quantum leap) is an electron changing from one energy...8 KB (868 words) - 14:59, 25 June 2024
- mechanics. It was proposed early in the 20th century. In molecular orbital theory, electrons in a molecule are not assigned to individual chemical bonds...22 KB (2,943 words) - 17:32, 23 June 2024
- In chemistry, a molecular orbital (/ɒrbədl/) is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This...35 KB (4,390 words) - 11:31, 6 June 2024
- The 18-electron rule is a chemical rule of thumb used primarily for predicting and rationalizing formulas for stable transition metal complexes, especially...17 KB (1,923 words) - 02:05, 19 June 2024
- main-group element, a valence electron can exist only in the outermost electron shell; for a transition metal, a valence electron can also be in an inner shell...24 KB (2,333 words) - 22:12, 20 May 2024
- VSEPR theory (redirect from Valence shell electron pair repulsion)VSEPR-predicted molecular geometry of a molecule is the one that has as little of this repulsion as possible. Gillespie has emphasized that the electron-electron repulsion...45 KB (4,038 words) - 13:09, 14 April 2024
- be estimated at a few electron volts. This is the case for most low-lying molecular energy states, and corresponds to transitions in the visible and ultraviolet...9 KB (988 words) - 21:45, 20 May 2024
- electron count or number of d electrons is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition...14 KB (1,701 words) - 03:27, 4 October 2023
- Energy level (redirect from Molecular energy state)Examples Molecular orbital diagrams, Jablonski diagrams, and Franck–Condon diagrams. Electrons in atoms and molecules can change (make transitions in) energy...22 KB (2,831 words) - 03:08, 12 February 2024
- The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline...58 KB (6,792 words) - 08:05, 9 July 2024
- their unpaired d electrons, as are many of their compounds. All of the elements that are ferromagnetic near room temperature are transition metals (iron,...40 KB (4,504 words) - 07:15, 17 February 2024
- Exciton (section Atomic and molecular excitons)An electron and an electron hole that are attracted to each other by the Coulomb force can form a bound state called an exciton. It is an electrically...35 KB (4,613 words) - 18:41, 4 June 2024
- be described visually, in a molecular orbital diagram. In the standard Diels–Alder, the electron rich diene has molecular orbitals that are higher in...26 KB (2,643 words) - 15:04, 9 March 2024
- three atomic orbitals form three molecular orbitals: one bonding, one non-bonding, and one anti-bonding. The two electrons go into the bonding orbital, resulting...5 KB (688 words) - 12:00, 26 March 2023
- of atomic orbitals combine to form the same number of molecular orbitals, although the electrons involved may be redistributed among the orbitals. This...38 KB (4,966 words) - 02:30, 13 March 2024
- Hyperfine structure (redirect from Hyperfine transition)other ways, in atomic and molecular spectra and in electron paramagnetic resonance spectra of free radicals and transition-metal ions. As the hyperfine...28 KB (4,377 words) - 18:33, 28 June 2024
- of AMO that studies atoms as an isolated system of electrons and an atomic nucleus, while molecular physics is the study of the physical properties of...25 KB (2,966 words) - 22:59, 22 April 2024
- The electron ( e− , or β− in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first...153 KB (15,428 words) - 02:28, 20 June 2024
- Transition from Electrons to Molecules 117. The main additional result derived from this second-order discussion is that if we assume all molecular forces
- of nuclei with their transitions at radio frequencies is used for a variety of applications, ranging from the studies of molecular structure to magnetic
- internal energy transitions. The position of spectral lines reveals various molecular parameters such as internuclear spacing and molecular bond angles.