Search results
Appearance
There is a page named "LiAlH4" on Wikipedia
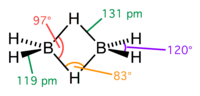
- Lithium aluminium hydride (redirect from Lialh4)abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger...35 KB (3,052 words) - 14:36, 12 July 2024
- Lithium (redirect from Li (element))hydrides, such as lithium aluminium hydride (LiAlH4), are used as high-energy additives to rocket propellants. LiAlH4 can also be used by itself as a solid fuel...140 KB (13,736 words) - 13:39, 20 July 2024
- advancement of catalytic technologies. Metal hydrides, such as MgH2, NaAlH4, LiAlH4, LiH, LaNi5H6, TiFeH2, ammonia borane, and palladium hydride represent...134 KB (14,154 words) - 19:47, 8 July 2024
- and also compounds that contain the hydride H− ion, those being NaH, LiH, LiAlH4 and CaH2. Some elements and compounds can be both reducing or oxidizing...15 KB (1,903 words) - 19:02, 8 July 2024
- Aluminium–lithium alloys (redirect from Aluminum-Lithium (Al-Li))Aluminium–lithium alloys (Al–Li alloys) are a set of alloys of aluminium and lithium, often also including copper and zirconium. Since lithium is the...30 KB (2,482 words) - 02:54, 9 July 2024
- borohydride. Both methods result in as much as 30% yield: 4 BCl3 + 3 LiAlH4 → 2 B2H6 + 3 LiAlCl4 4 BF3 + 3 NaBH4 → 2 B2H6 + 3 NaBF4 When heated with NaBH4, tin(II)...27 KB (2,596 words) - 17:47, 18 June 2024
- Sodium aluminium hydride (redirect from NaAlH4)chloride to give the popular reagent lithium aluminium hydride: LiCl + NaAlH4 → LiAlH4 + NaCl The compound reacts rapidly, even violently, with protic reagents...5 KB (456 words) - 11:59, 14 April 2024
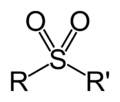
- groups can be reduced to the sulfide with DIBALH. Lithium aluminium hydride (LiAlH4) reduces some but not all sulfones to sulfides.: 1851 In inorganic chemistry...2 KB (201 words) - 20:23, 29 April 2023
- readily.[citation needed] Hydride transfer reagents, such as NaBH4 and LiAlH4, reduce by atom transfer: they transfer the equivalent of hydride or H−...37 KB (3,583 words) - 17:38, 9 July 2024
- Lithium hydride (redirect from LiH)g., 8 LiH + Al2Cl6 → 2 Li[AlH4] + 6 LiCl 2 LiH + B2H6 → 2 Li[BH4] With a hydrogen content in proportion to its mass three times that of NaH, LiH has the...19 KB (1,928 words) - 01:12, 12 June 2024
- Aluminium hydride (redirect from AlH3)Li[AlH4]. Several other methods exist for the preparation of aluminium hydride: 2 Li[AlH4] + BeCl2 → 2 AlH3 + Li2[BeH2Cl2] 2 Li[AlH4] + H2SO4 → 2 AlH3...31 KB (3,203 words) - 20:53, 27 May 2024
- Pt , Pd , BH 3 , LiAlH 4 or other hydrides C 2 H 5 OH ethanol {\displaystyle {\ce {(CH2CH2)O{}+H2->[{} \atop {\ce {Ni,Pt,Pd,BH3,LiAlH4}}{\text{ or other...108 KB (11,539 words) - 06:12, 9 July 2024
- α-β unsaturated esters to the corresponding allylic alcohol. By contrast, LiAlH4 reduces esters and acyl chlorides to primary alcohols, and nitriles to primary...8 KB (574 words) - 23:25, 11 June 2024
- Tosylates can be cleaved with lithium aluminium hydride: 4 CH3C6H4SO2OR + LiAlH4 → LiAl(O3SC6H4CH3)4 + 4 RH Thus, tosylation followed by reduction allows for...6 KB (432 words) - 20:02, 9 November 2022
- N-Butyllithium (redirect from N-BuLi)n-Butyllithium C4H9Li (abbreviated n-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers...18 KB (1,856 words) - 21:35, 4 March 2024
- chloride. 4 LiH lithium hydride + AlCl 3 ⟶ LiAlH 4 + 3 LiCl {\displaystyle {\ce {{\overset {lithium\\ hydride}{4 LiH}}+ AlCl3 -> LiAlH4{}+ 3 LiCl}}} According...21 KB (2,333 words) - 12:44, 22 May 2024
- Sodium bis(2-methoxyethoxy)aluminium hydride (redirect from Red-Al)As a reagent, SMEAH is comparable with lithium aluminium hydride (LAH, LiAlH4). It is a safer alternative to LAH and related hydrides. SMEAH exhibits...5 KB (381 words) - 19:13, 13 April 2024
- The McMurry reaction originally involved the use of a mixture TiCl3 and LiAlH4, which produces the active reagents. Related species have been developed...7 KB (758 words) - 19:29, 27 October 2022
- .... Zn2SiO4 .... R4 Dioptase R4 - M H4 6 ls 25 Scapolite - - - 'inNa A1 si 0 c11r ° ' T3 Vesuvianite H2Ca5(Al, Fe)3Si5O1g T Zircon .. ZrSiO4 . .. T
- by nucleophilic additions. Aldehydes and ketones are reduced by NaBH4 or LiAlH4 to yield primary and secondary alcohols, respectively. Addition of Grignard
- RCO2-Na+ + H2O RCO2H + NaHCO3 ----> RCO2-Na+ + H2O + CO2 2) Reduction: RCO2H + LiAlH4 --- (1) Et2O -- (2) H2O ----> RCH2OH 2a) Fukyama reduction: Pd and Et3SiH