L-DOPA
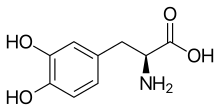 Skeletal formula of L-DOPA
| |
 | |
| Names | |
|---|---|
| IUPAC name
(S)-2-Amino-3-(3,4-dihydroxyphenyl)propanoic acid
| |
| Other names
l-3,4-Dihydroxyphenylalanine; Levodopa
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.405 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H11NO4 | |
| Molar mass | 197.19 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
l-DOPA, also known as l-3,4-dihydroxyphenylalanine and used medically as levodopa, is made and used as part of the normal biology of some plants[2] and animals, including humans. Humans, as well as a portion of the other animals that utilize l-DOPA, make it via biosynthesis from the amino acid l-tyrosine.
l-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, l-DOPA itself mediates neurotrophic factor release by the brain and CNS.[3][4] In some plant families (of the order Caryophyllales), l-DOPA is the central precursor of a biosynthetic pathway that produces a class of pigments called betalains.[5]
l-DOPA can be manufactured and in its pure form is sold as a drug with the INN levodopa. Trade names include Sinemet, Pharmacopa, Atamet, and Stalevo. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia.
l-DOPA has a counterpart with opposite chirality, d-DOPA. As is true for many molecules, the human body produces only one of these isomers (the l-DOPA form). The enantiomeric purity of l-DOPA may be analyzed by determination of the optical rotation or by chiral thin-layer chromatography.[6]
Biological role
l-DOPA is produced from the amino acid l-tyrosine by the enzyme tyrosine hydroxylase. l-DOPA can act as an l-tyrosine mimetic and be incorporated into proteins by mammalian cells in place of l-tyrosine, generating protease-resistant and aggregate-prone proteins in vitro and may contribute to neurotoxicity with chronic l-DOPA administration.[10] It is also the precursor for the monoamine or catecholamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline). Dopamine is formed by the decarboxylation of l-DOPA by aromatic l-amino acid decarboxylase (AADC).
l-DOPA can be directly metabolized by catechol-O-methyl transferase to 3-O-methyldopa, and then further to vanillactic acid. This metabolic pathway is nonexistent in the healthy body, but becomes important after peripheral l-DOPA administration in patients with Parkinson's disease or in the rare cases of patients with AADC enzyme deficiency.[11]
l-Phenylalanine, l-tyrosine, and l-DOPA are all precursors to the biological pigment melanin. The enzyme tyrosinase catalyzes the oxidation of l-DOPA to the reactive intermediate dopaquinone, which reacts further, eventually leading to melanin oligomers. In addition, tyrosinase can convert tyrosine directly to l-DOPA in the presence of a reducing agent such as ascorbic acid.[12]
History
l-dopa was first islolated from the seeds of the Vicia faba or broad bean plant in 1913 by Swiss biochemist Markus Guggenheim.[13]
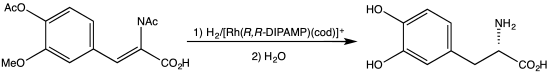
The 2001 Nobel Prize in Chemistry was also related to l-DOPA: the Nobel Committee awarded one-quarter of the prize to William S. Knowles for his work on chirally catalysed hydrogenation reactions, the most noted example of which was used for the synthesis of l-DOPA.[14][15][16]
Other organisms
Marine adhesion
l-DOPA is a key compound in the formation of marine adhesive proteins, such as those found in mussels.[17][18] It is believed to be responsible for the water-resistance and rapid curing abilities of these proteins. l-DOPA may also be used to prevent surfaces from fouling by bonding antifouling polymers to a susceptible substrate.[19] The versatile chemistry of l-DOPA can be exploited in nanotechnology.[20] For example, DOPA-containing self-assembling peptides were found to form functional nanostructures, adhesives and gels.[21][22][23][24]
Plants and in the environment
In plants, L-DOPA functions as an allelochemical which inhibits the growth of certain species, and is produced and secreted by a few legume species such as the broad bean Vicia faba and the velvet bean Mucuna pruriens.[25] Its effect is strongly dependent on the pH and the reactivity of iron in the soil.[26]
Use as a medication and supplement
L-DOPA is used medically under the name levodopa in the treatment of Parkinson's disease and certain other medical conditions. It is usually used in combination with a peripherally selective aromatic L-amino acid decarboxylase (AAAD) inhibitor such as carbidopa or benserazide. These agents increase the strength and duration of levodopa. Combination formulations include levodopa/carbidopa and levodopa/benserazide, as well as levodopa/carbidopa/entacapone.
L-DOPA is found in high amounts in Mucuna pruriens (velvet bean) and is available and used over-the-counter as a supplement.
References
- ^ Howard ST, Hursthouse MB, Lehmann CW, Poyner EA (1995). "Experimental and theoretical determination of electronic properties in Ldopa". Acta Crystallogr. B. 51 (3): 328–337. Bibcode:1995AcCrB..51..328H. doi:10.1107/S0108768194011407. S2CID 96802274.
- ^ Cohen PA, Avula B, Katragunta K, Khan I (October 2022). "Levodopa Content of Mucuna pruriens Supplements in the NIH Dietary Supplement Label Database". JAMA Neurology. 79 (10): 1085–1086. doi:10.1001/jamaneurol.2022.2184. PMC 9361182. PMID 35939305.
- ^ Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS (September 2008). "L-DOPA is an endogenous ligand for OA1". PLOS Biology. 6 (9): e236. doi:10.1371/journal.pbio.0060236. PMC 2553842. PMID 18828673.
- ^ Hiroshima Y, Miyamoto H, Nakamura F, Masukawa D, Yamamoto T, Muraoka H, et al. (January 2014). "The protein Ocular albinism 1 is the orphan GPCR GPR143 and mediates depressor and bradycardic responses to DOPA in the nucleus tractus solitarii". British Journal of Pharmacology. 171 (2): 403–14. doi:10.1111/bph.12459. PMC 3904260. PMID 24117106.
- ^ Polturak G, Breitel D, Grossman N, Sarrion-Perdigones A, Weithorn E, Pliner M, et al. (2016). "Elucidation of the first committed step in betalain biosynthesis enables the heterologous engineering of betalain pigments in plants". New Phytol. 210 (1): 269–283. doi:10.1111/nph.13796. PMID 26683006.
- ^ Martens J, Günther K, Schickedanz M (1986). "Resolution of Optical Isomers by Thin-Layer Chromatography: Enantiomeric Purity of Methyldopa". Arch. Pharm. 319 (6): 572–574. doi:10.1002/ardp.19863190618. S2CID 97903386.
- ^ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ^ Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ^ Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Journal of Pharmacology. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- ^ Rodgers KJ (March 2014). "Non-protein amino acids and neurodegeneration: the enemy within". Experimental Neurology. 253: 192–196. doi:10.1016/j.expneurol.2013.12.010. PMID 24374297. S2CID 2288729.
- ^ Hyland K, Clayton PT (December 1992). "Aromatic L-amino acid decarboxylase deficiency: diagnostic methodology" (PDF). Clinical Chemistry. 38 (12): 2405–10. doi:10.1093/clinchem/38.12.2405. PMID 1281049. Archived from the original (PDF) on 7 June 2011. Retrieved 16 October 2008.
- ^ Ito S, Kato T, Shinpo K, Fujita K (September 1984). "Oxidation of tyrosine residues in proteins by tyrosinase. Formation of protein-bonded 3,4-dihydroxyphenylalanine and 5-S-cysteinyl-3,4-dihydroxyphenylalanine". The Biochemical Journal. 222 (2): 407–11. doi:10.1042/bj2220407. PMC 1144193. PMID 6433900.
- ^ Ovallath S, Sulthana B (2017). "Levodopa: History and Therapeutic Applications". Annals of Indian Academy of Neurology. 20 (3): 185–189. doi:10.4103/aian.AIAN_241_17. PMC 5586109. PMID 28904446.
- ^ Knowles WS (1983). "Asymmetric hydrogenation". Accounts of Chemical Research. 16 (3): 106–112. doi:10.1021/ar00087a006.
- ^ "Synthetic scheme for total synthesis of DOPA, L- (Monsanto)". UW Madison, Department of Chemistry. Retrieved 30 September 2013.
- ^ Knowles WS (March 1986). "Application of organometallic catalysis to the commercial production of L-DOPA". Journal of Chemical Education. 63 (3): 222. Bibcode:1986JChEd..63..222K. doi:10.1021/ed063p222.
- ^ Waite JH, Andersen NH, Jewhurst S, Sun C (2005). "Mussel Adhesion: Finding the Tricks Worth Mimicking". J Adhesion. 81 (3–4): 1–21. doi:10.1080/00218460590944602. S2CID 136967853.
- ^ "Study Reveals Details Of Mussels' Tenacious Bonds". Science Daily. 16 August 2006. Retrieved 30 September 2013.
- ^ "Mussel Adhesive Protein Mimetics". Archived from the original on 29 May 2006.
- ^ Giuri D, Ravarino P, Tomasini C (June 2021). "L-Dopa in small peptides: an amazing functionality to form supramolecular materials". Organic & Biomolecular Chemistry. 19 (21): 4622–4636. doi:10.1039/D1OB00378J. hdl:11585/840774. PMID 33978030. S2CID 234474122.
- ^ Fichman G, Adler-Abramovich L, Manohar S, Mironi-Harpaz I, Guterman T, Seliktar D, et al. (July 2014). "Seamless metallic coating and surface adhesion of self-assembled bioinspired nanostructures based on di-(3,4-dihydroxy-L-phenylalanine) peptide motif". ACS Nano. 8 (7): 7220–7228. doi:10.1021/nn502240r. PMC 4108209. PMID 24936704.
- ^ Fichman G, Guterman T, Adler-Abramovich L, Gazit E (August 2014). "The Use of the Calcitonin Minimal Recognition Module for the Design of DOPA-Containing Fibrillar Assemblies". Nanomaterials. 4 (3): 726–740. doi:10.3390/nano4030726. PMC 5304689. PMID 28344244.
- ^ Fichman G, Andrews C, Patel NL, Schneider JP (October 2021). "Antibacterial Gel Coatings Inspired by the Cryptic Function of a Mussel Byssal Peptide". Advanced Materials. 33 (40): e2103677. Bibcode:2021AdM....3303677F. doi:10.1002/adma.202103677. PMC 8492546. PMID 34423482.
- ^ Maity S, Nir S, Zada T, Reches M (October 2014). "Self-assembly of a tripeptide into a functional coating that resists fouling". Chemical Communications. 50 (76): 11154–11157. doi:10.1039/C4CC03578J. PMID 25110984.
- ^ Fujii Y, Shibuya T, Yasuda T (1991). "L-3,4-Dihydroxyphenylalanine as an Allelochemical Candidate from Mucuna pruriens (L.) DC. var. utilis". Agricultural and Biological Chemistry. 55 (2): 617–618. doi:10.1080/00021369.1991.10870627.
- ^ Hsieh EJ, Liao SW, Chang CY, Tseng CH, Wang SL, Grillet L (2023). "L-DOPA induces iron accumulation in roots of Ipomoea aquatica and Arabidopsis thaliana in a pH-dependent manner". Botanical Studies. 64 (24): 617–618. Bibcode:2023BotSt..64...24H. doi:10.1186/s40529-023-00396-7. PMC 10449704. PMID 37620733.