History of virology
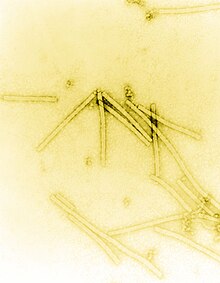
The history of virology – the scientific study of viruses and the infections they cause – began in the closing years of the 19th century. Although Edward Jenner and Louis Pasteur developed the first vaccines to protect against viral infections, they did not know that viruses existed. The first evidence of the existence of viruses came from experiments with filters that had pores small enough to retain bacteria. In 1892, Dmitri Ivanovsky used one of these filters to show that sap from a diseased tobacco plant remained infectious to healthy tobacco plants despite having been filtered. Martinus Beijerinck called the filtered, infectious substance a "virus" and this discovery is considered to be the beginning of virology.
The subsequent discovery and partial characterization of bacteriophages by Frederick Twort and Félix d'Herelle further catalyzed the field, and by the early 20th century many viruses had been discovered. In 1926, Thomas Milton Rivers defined viruses as obligate parasites. Viruses were demonstrated to be particles, rather than a fluid, by Wendell Meredith Stanley, and the invention of the electron microscope in 1931 allowed their complex structures to be visualised.
Pioneers


Despite his other successes, Louis Pasteur (1822–1895) was unable to find a causative agent for rabies and speculated about a pathogen too small to be detected using a microscope.[1] In 1884, the French microbiologist Charles Chamberland (1851–1931) invented a filter – known today as the Chamberland filter – that had pores smaller than bacteria. Thus, he could pass a solution containing bacteria through the filter and completely remove them from the solution.[2]
In 1876, Adolf Mayer, who directed the Agricultural Experimental Station in Wageningen, was the first to show that what he called "Tobacco Mosaic Disease" was infectious. He thought that it was caused by either a toxin or a very small bacterium. Later, in 1892, the Russian biologist Dmitry Ivanovsky (1864–1920) used a Chamberland filter to study what is now known as the tobacco mosaic virus. His experiments showed that crushed leaf extracts from infected tobacco plants remain infectious after filtration. Ivanovsky suggested the infection might be caused by a toxin produced by bacteria, but did not pursue the idea.[3]
In 1898, the Dutch microbiologist Martinus Beijerinck (1851–1931), a microbiology teacher at the Agricultural School in Wageningen repeated experiments by Adolf Mayer and became convinced that filtrate contained a new form of infectious agent.[4] He observed that the agent multiplied only in cells that were dividing and he called it a contagium vivum fluidum (soluble living germ) and re-introduced the word virus.[3] Beijerinck maintained that viruses were liquid in nature, a theory later discredited by the American biochemist and virologist Wendell Meredith Stanley (1904–1971), who proved that they were in fact, particles.[3] In the same year, 1898, Friedrich Loeffler (1852–1915) and Paul Frosch (1860–1928) passed the first animal virus through a similar filter and discovered the cause of foot-and-mouth disease.[5]
The first human virus to be identified was the yellow fever virus.[6] In 1881, Carlos Finlay (1833–1915), a Cuban physician, first conducted and published research that indicated that mosquitoes were carrying the cause of yellow fever,[7] a theory proved in 1900 by commission headed by Walter Reed (1851–1902). During 1901 and 1902, William Crawford Gorgas (1854–1920) organised the destruction of the mosquitoes' breeding habitats in Cuba, which dramatically reduced the prevalence of the disease.[8] Gorgas later organised the elimination of the mosquitoes from Panama, which allowed the Panama Canal to be opened in 1914.[9] The virus was finally isolated by Max Theiler (1899–1972) in 1932 who went on to develop a successful vaccine.[10]
By 1928 enough was known about viruses to enable the publication of Filterable Viruses, a collection of essays covering all known viruses edited by Thomas Milton Rivers (1888–1962). Rivers, a survivor of typhoid fever contracted at the age of twelve, went on to have a distinguished career in virology. In 1926, he was invited to speak at a meeting organised by the Society of American Bacteriology where he said for the first time, "Viruses appear to be obligate parasites in the sense that their reproduction is dependent on living cells."[11]
The notion that viruses were particles was not considered unnatural and fitted in nicely with the germ theory. It is assumed that Dr. J. Buist of Edinburgh was the first person to see virus particles in 1886, when he reported seeing "micrococci" in vaccine lymph, though he had probably observed clumps of vaccinia.[12] In the years that followed, as optical microscopes were improved "inclusion bodies" were seen in many virus-infected cells, but these aggregates of virus particles were still too small to reveal any detailed structure. It was not until the invention of the electron microscope in 1931 by the German engineers Ernst Ruska (1906–1988) and Max Knoll (1887–1969),[13] that virus particles, especially bacteriophages, were shown to have complex structures. The sizes of viruses determined using this new microscope fitted in well with those estimated by filtration experiments. Viruses were expected to be small, but the range of sizes came as a surprise. Some were only a little smaller than the smallest known bacteria, and the smaller viruses were of similar sizes to complex organic molecules.[14]
In 1935, Wendell Stanley examined the tobacco mosaic virus and found it was mostly made of protein.[15] In 1939, Stanley and Max Lauffer (1914) separated the virus into protein and nucleic acid,[16] which was shown by Stanley's postdoctoral fellow Hubert S. Loring to be specifically RNA.[17] The discovery of RNA in the particles was important because in 1928, Fred Griffith (c. 1879–1941) provided the first evidence that its "cousin", DNA, formed genes.[18]
In Pasteur's day, and for many years after his death, the word "virus" was used to describe any cause of infectious disease. Many bacteriologists soon discovered the cause of numerous infections. However, some infections remained, many of them horrendous, for which no bacterial cause could be found. These agents were invisible and could only be grown in living animals. The discovery of viruses paved the way to understanding these mysterious infections. And, although Koch's postulates could not be fulfilled for many of these infections, this did not stop the pioneer virologists from looking for viruses in infections for which no other cause could be found.[19]
Bacteriophages

Discovery
Bacteriophages are the viruses that infect and replicate in bacteria. They were discovered in the early 20th century, by the English bacteriologist Frederick Twort (1877–1950).[20] But before this time, in 1896, the bacteriologist Ernest Hanbury Hankin (1865–1939) reported that something in the waters of the River Ganges could kill Vibrio cholerae – the cause of cholera. The agent in the water could be passed through filters that remove bacteria but was destroyed by boiling.[21] Twort discovered the action of bacteriophages on staphylococci bacteria. He noticed that when grown on nutrient agar some colonies of the bacteria became watery. He collected some of these watery colonies and passed them through a Chamberland filter to remove the bacteria and discovered that when the filtrate was added to fresh cultures of bacteria, they in turn became watery.[20] He proposed that the agent might be "an amoeba, an ultramicroscopic virus, a living protoplasm, or an enzyme with the power of growth".[21]
Félix d'Herelle (1873–1949) was a mainly self-taught French-Canadian microbiologist. In 1917 he discovered that "an invisible antagonist", when added to bacteria on agar, would produce areas of dead bacteria.[20] The antagonist, now known to be a bacteriophage, could pass through a Chamberland filter. He accurately diluted a suspension of these viruses and discovered that the highest dilutions (lowest virus concentrations), rather than killing all the bacteria, formed discrete areas of dead organisms. Counting these areas and multiplying by the dilution factor allowed him to calculate the number of viruses in the original suspension.[22] He realised that he had discovered a new form of virus and later coined the term "bacteriophage".[23][24] Between 1918 and 1921 d'Herelle discovered different types of bacteriophages that could infect several other species of bacteria including Vibrio cholerae.[25] Bacteriophages were heralded as a potential treatment for diseases such as typhoid and cholera, but their promise was forgotten with the development of penicillin.[23] Since the early 1970s, bacteria have continued to develop resistance to antibiotics such as penicillin, and this has led to a renewed interest in the use of bacteriophages to treat serious infections.[26]
1920–1940: Early research
D'Herelle travelled widely to promote the use of bacteriophages in the treatment of bacterial infections. In 1928, he became professor of biology at Yale and founded several research institutes.[27] He was convinced that bacteriophages were viruses despite opposition from established bacteriologists such as the Nobel Prize winner Jules Bordet (1870–1961). Bordet argued that bacteriophages were not viruses but just enzymes released from "lysogenic" bacteria. He said "the invisible world of d'Herelle does not exist".[28] But in the 1930s, the proof that bacteriophages were viruses was provided by Christopher Andrewes (1896–1988) and others. They showed that these viruses differed in size and in their chemical and serological properties. In 1940, the first electron micrograph of a bacteriophage was published and this silenced sceptics who had argued that bacteriophages were relatively simple enzymes and not viruses.[29] Numerous other types of bacteriophages were quickly discovered and were shown to infect bacteria wherever they are found. Early research was interrupted by World War II. d'Herelle, despite his Canadian citizenship, was interned by the Vichy Government until the end of the war.[30]
Modern era
Knowledge of bacteriophages increased in the 1940s following the formation of the Phage Group by scientists throughout the US. Among the members were Max Delbrück (1906–1981) who founded a course on bacteriophages at Cold Spring Harbor Laboratory.[26] Other key members of the Phage Group included Salvador Luria (1912–1991) and Alfred Hershey (1908–1997). During the 1950s, Hershey and Chase made important discoveries on the replication of DNA during their studies on a bacteriophage called T2. Together with Delbruck they were jointly awarded the 1969 Nobel Prize in Physiology or Medicine "for their discoveries concerning the replication mechanism and the genetic structure of viruses".[31] Since then, the study of bacteriophages has provided insights into the switching on and off of genes, and a useful mechanism for introducing foreign genes into bacteria and many other fundamental mechanisms of molecular biology.[32]
Plant viruses
In 1882, Adolf Mayer (1843–1942) described a condition of tobacco plants, which he called "mosaic disease" ("mozaïkziekte"). The diseased plants had variegated leaves that were mottled.[33] He excluded the possibility of a fungal infection and could not detect any bacterium and speculated that a "soluble, enzyme-like infectious principle was involved".[34] He did not pursue his idea any further, and it was the filtration experiments of Ivanovsky and Beijerinck that suggested the cause was a previously unrecognised infectious agent. After tobacco mosaic was recognized as a virus disease, virus infections of many other plants were discovered.[34]
The importance of tobacco mosaic virus in the history of viruses cannot be overstated. It was the first virus to be discovered, and the first to be crystallised and its structure shown in detail. The first X-ray diffraction pictures of the crystallised virus were obtained by Bernal and Fankuchen in 1941. On the basis of her pictures, Rosalind Franklin discovered the full structure of the virus in 1955.[35] In the same year, Heinz Fraenkel-Conrat and Robley Williams showed that purified tobacco mosaic virus RNA and its coat protein can assemble by themselves to form functional viruses, suggesting that this simple mechanism was probably the means through which viruses were created within their host cells.[36]
By 1935, many plant diseases were thought to be caused by viruses. In 1922, John Kunkel Small (1869–1938) discovered that insects could act as vectors and transmit virus to plants. In the following decade many diseases of plants were shown to be caused by viruses that were carried by insects and in 1939, Francis Holmes, a pioneer in plant virology,[37] described 129 viruses that caused disease of plants.[38] Modern, intensive agriculture provides a rich environment for many plant viruses. In 1948, in Kansas, US, 7% of the wheat crop was destroyed by wheat streak mosaic virus. The virus was spread by mites called Aceria tulipae.[39]
In 1970, the Russian plant virologist Joseph Atabekov discovered that many plant viruses only infect a single species of host plant.[37] The International Committee on Taxonomy of Viruses now recognises over 900 plant viruses.[40]
20th century
By the end of the 19th century, viruses were defined in terms of their infectivity, their ability to be filtered, and their requirement for living hosts. Up until this time, viruses had only been grown in plants and animals, but in 1906, Ross Granville Harrison (1870–1959) invented a method for growing tissue in lymph,[41] and, in 1913, E Steinhardt, C Israeli, and RA Lambert used this method to grow vaccinia virus in fragments of guinea pig corneal tissue.[42] In 1928, HB and MC Maitland grew vaccinia virus in suspensions of minced hens' kidneys.[43] Their method was not widely adopted until the 1950s, when poliovirus was grown on a large scale for vaccine production.[44] In 1941–42, George Hirst (1909–94) developed assays based on haemagglutination to quantify a wide range of viruses as well as virus-specific antibodies in serum.[45][46]
Influenza

Although the influenza virus that caused the 1918–1919 influenza pandemic was not discovered until the 1930s, the descriptions of the disease and subsequent research has proved it was to blame.[47] The pandemic killed 40–50 million people in less than a year,[48] but the proof that it was caused by a virus was not obtained until 1933.[49] Haemophilus influenzae is an opportunistic bacterium which commonly follows influenza infections; this led the eminent German bacteriologist Richard Pfeiffer (1858–1945) to incorrectly conclude that this bacterium was the cause of influenza.[50] A major breakthrough came in 1931, when the American pathologist Ernest William Goodpasture grew influenza and several other viruses in fertilised chickens' eggs.[51] Hirst identified an enzymic activity associated with the virus particle, later characterised as the neuraminidase, the first demonstration that viruses could contain enzymes. Frank Macfarlane Burnet showed in the early 1950s that the virus recombines at high frequencies, and Hirst later deduced that it has a segmented genome.[52]
Poliomyelitis
In 1949, John F. Enders (1897–1985) Thomas Weller (1915–2008), and Frederick Robbins (1916–2003) grew polio virus for the first time in cultured human embryo cells, the first virus to be grown without using solid animal tissue or eggs. Infections by poliovirus most often cause the mildest of symptoms. This was not known until the virus was isolated in cultured cells and many people were shown to have had mild infections that did not lead to poliomyelitis. But, unlike other viral infections, the incidence of polio – the rarer severe form of the infection – increased in the 20th century and reached a peak around 1952. The invention of a cell culture system for growing the virus enabled Jonas Salk (1914–1995) to make an effective polio vaccine.[53]
Epstein–Barr virus
Denis Parsons Burkitt (1911–1993) was born in Enniskillen, County Fermanagh, Ireland. He was the first to describe a type of cancer that now bears his name Burkitt's lymphoma. This type of cancer was endemic in equatorial Africa and was the commonest malignancy of children in the early 1960s.[54] In an attempt to find a cause for the cancer, Burkitt sent cells from the tumour to Anthony Epstein (b. 1921) a British virologist, who along with Yvonne Barr and Bert Achong (1928–1996), and after many failures, discovered viruses that resembled herpes virus in the fluid that surrounded the cells. The virus was later shown to be a previously unrecognised herpes virus, which is now called Epstein–Barr virus.[55] Surprisingly, Epstein–Barr virus is a very common but relatively mild infection of Europeans. Why it can cause such a devastating illness in Africans is not fully understood, but reduced immunity to virus caused by malaria might be to blame.[56] Epstein–Barr virus is important in the history of viruses for being the first virus shown to cause cancer in humans.[57]
Late 20th and early 21st century

The second half of the 20th century was the golden age of virus discovery and most of the 2,000 recognised species of animal, plant, and bacterial viruses were discovered during these years.[58][59] In 1946, bovine virus diarrhea was discovered,[60] which is still possibly the most common pathogen of cattle throughout the world[61] and in 1957, equine arterivirus was discovered.[62] In the 1950s, improvements in virus isolation and detection methods resulted in the discovery of several important human viruses including varicella zoster virus,[63] the paramyxoviruses[64] – which include measles virus[65] and respiratory syncytial virus[64] – and the rhinoviruses that cause the common cold.[66] In the 1960s more viruses were discovered. In 1963, the hepatitis B virus was discovered by Baruch Blumberg (b. 1925).[67] Reverse transcriptase, the key enzyme that retroviruses use to translate their RNA into DNA, was first described in 1970, independently by Howard Temin and David Baltimore (b. 1938).[68] This was important to the development of antiviral drugs – a key turning-point in the history of viral infections.[69] In 1983, Luc Montagnier (b. 1932) and his team at the Pasteur Institute in France first isolated the retrovirus now called HIV.[70] In 1989 Michael Houghton's team at Chiron Corporation discovered hepatitis C.[71] New viruses and strains of viruses were discovered in every decade of the second half of the 20th century. These discoveries have continued in the 21st century as new viral diseases such as SARS[72] and nipah virus[73] have emerged. Despite scientists' achievements over the past one hundred years, viruses continue to pose new threats and challenges.[74]
See also
References
- ^ Bordenave G (May 2003). "Louis Pasteur (1822–1895)". Microbes and Infection / Institut Pasteur. 5 (6): 553–60. doi:10.1016/S1286-4579(03)00075-3. PMID 12758285.
- ^ Shors 2008, pp. 76–77
- ^ a b c Topley & Wilson 1998, p. 3
- ^ Leppard, Keith, Nigel Dimmock, Easton, Andrew (2007). Introduction to Modern Virology. Blackwell Publishing Limited. pp. 4–5. ISBN 978-1-4051-3645-7.
- ^ Fenner F (2009). "History of Virology: Vertebrate Viruses". In Mahy B, Van Regenmortal M (eds.). Desk Encyclopedia of General Virology. Oxford, UK: Academic Press. p. 15. ISBN 978-0-12-375146-1.
- ^ Staples JE, Monath TP (27 August 2008). "Yellow fever: 100 years of discovery". JAMA: The Journal of the American Medical Association. 300 (8): 960–2. doi:10.1001/jama.300.8.960. PMID 18728272.
- ^ Chiong MA (December 1989). "Dr. Carlos Finlay and yellow fever". Canadian Medical Association Journal. 141 (11): 1126. PMC 1451274. PMID 2684378.
- ^ Litsios S (2001). "William Crawford Gorgas (1854–1920)". Perspectives in Biology and Medicine. 44 (3): 368–78. doi:10.1353/pbm.2001.0051. PMC 1089739. PMID 11482006.
- ^ Patterson R (September 1989). "Dr. William Gorgas and his war with the mosquito". Canadian Medical Association Journal. 141 (6): 596–7, 599. PMC 1451363. PMID 2673502.
- ^ Frierson JG (June 2010). "The yellow fever vaccine: a history". Yale Journal of Biology and Medicine. 83 (2): 77–85. PMC 2892770. PMID 20589188.
- ^ Horsfall FL (1965). "Thomas Milton Rivers, September 3, 1888–May 12, 1962" (PDF). Biogr Mem Natl Acad Sci. 38: 263–94. PMID 11615452.
- ^ *In 1887, Buist visualised one of the largest, Vaccinia virus, by optical microscopy after staining it. Vaccinia was not known to be a virus at that time. Buist J (1887). Vaccinia and Variola: a study of their life history. London: Churchill.
- ^ From Ekspång G, ed. (1993). Nobel Lectures, Physics 1981–1990. World Scientific. ISBN 978-9810207281.
- ^ Carr, N. G., Mahy, B. W. J., Pattison, J. R., Kelly, D. P. (1984). The Microbe 1984: Thirty-sixth Symposium of the Society for General Microbiology, held at the University of Warwick, April 1984. Symposia of the Society for general microbiology. Vol. 36. Cambridge University Press. p. 4. ISBN 978-0-521-26056-5. OCLC 499302635.
- ^ Stanley WM, Loring HS (1936). "The isolation of crystalline tobacco mosaic virus protein from diseased tomato plants". Science. 83 (2143): 85. Bibcode:1936Sci....83...85S. doi:10.1126/science.83.2143.85. PMID 17756690.
- ^ Stanley WM, Lauffer MA (1939). "Disintegration of tobacco mosaic virus in urea solutions". Science. 89 (2311): 345–347. Bibcode:1939Sci....89..345S. doi:10.1126/science.89.2311.345. PMID 17788438.
- ^ Loring HS (1939). "Properties and hydrolytic products of nucleic acid from tobacco mosaic virus". Journal of Biological Chemistry. 130 (1): 251–258. doi:10.1016/S0021-9258(18)73577-1.
- ^ Burton E. Tropp (2007). Molecular Biology: Genes to Proteins. Burton E. Tropp. Sudbury, Massachusetts: Jones & Bartlett Publishers. p. 12. ISBN 978-0-7637-5963-6.
- ^ The Microbe 1984, p. 3
- ^ a b c d e Shors T (2008). Understanding Viruses. Sudbury, Mass: Jones & Bartlett Publishers. p. 589. ISBN 978-0-7637-2932-5.
- ^ a b Ackermann HW (2009). "History of Virology: Bacteriophages". Desk Encyclopedia of General Virology. Academic Press. p. 3. ISBN 9780123751621.
- ^ a b D'Herelle F (September 2007). "On an invisible microbe antagonistic toward dysenteric bacilli: brief note by Mr. F. D'Herelle, presented by Mr. Roux☆". Research in Microbiology. 158 (7): 553–4. doi:10.1016/j.resmic.2007.07.005. PMID 17855060.
- ^ a b Ackermann HW (2009). "History of Virology: Bacteriophages". Desk Encyclopedia of General Virology. Academic Press. p. 4. ISBN 9780123751621.
- ^ "The antagonistic microbe can never be cultivated in media in the absence of the dysentery bacillus. It does not attack heat-killed dysentery bacilli, but is cultivated perfectly in a suspension of washed cells in physiological saline. This indicates that the anti dysentery microbe is an obligate bacteriophage". Felix d'Herelle (1917) An invisible microbe that is antagonistic to the dysentery bacillus (1917) Comptes rendus Acad. Sci. Paris Retrieved on 2 December 2010
- ^ Ackermann HW (2009). "History of Virology: Bacteriophages". Desk Encyclopedia of General Virology. Academic Press. p. 4 Table 1. ISBN 9780123751621.
- ^ a b Shors 2008, p. 591
- ^ Shors 2008, p. 590
- ^ Quoted in: Ackermann HW (2009). "History of Virology: Bacteriophages". Desk Encyclopedia of General Virology. Academic Press. p. 4. ISBN 9780123751621.
- ^ Ackermann HW (2009). "History of Virology: Bacteriophages". Desk Encyclopedia of General Virology. Academic Press. pp. 3–5. ISBN 9780123751621.
- ^ Ackermann HW (2009). "History of Virology: Bacteriophages". Desk Encyclopedia of General Virology. Academic Press. p. 5. ISBN 9780123751621.
- ^ Nobel Organisation
- ^ Ackermann HW (2009). "History of Virology: Bacteriophages". Desk Encyclopedia of General Virology. Academic Press. pp. 5–10 Table 1. ISBN 9780123751621.
- ^ Mayer A (1882) Over de moza¨ıkziekte van de tabak: voorloopige mededeeling. Tijdschr Landbouwkunde Groningen 2: 359–364 (In German)
- ^ a b Quoted in: van der Want JP, Dijkstra J (August 2006). "A history of plant virology". Archives of Virology. 151 (8): 1467–98. doi:10.1007/s00705-006-0782-3. PMID 16732421. S2CID 12180571.
- ^ Creager AN, Morgan GJ (June 2008). "After the double helix: Rosalind Franklin's research on Tobacco mosaic virus". Isis. 99 (2): 239–72. doi:10.1086/588626. PMID 18702397. S2CID 25741967.
- ^ Leppard, Keith, Nigel Dimmock, Easton, Andrew (2007). Introduction to Modern Virology. Blackwell Publishing Limited. p. 12. ISBN 978-1-4051-3645-7.
- ^ a b Pennazio S, Roggero P, Conti M (October 2001). "A history of plant virology. Mendelian genetics and resistance of plants to viruses". New Microbiology. 24 (4): 409–24. PMID 11718380.
- ^ Shors 2008, p. 563
- ^ Hansing D, Johnston C, Melchers L, Fellows H (1949). "Kansas Phytopathological Notes". Transactions of the Kansas Academy of Science. 52 (3): 363–369. doi:10.2307/3625805. JSTOR 3625805.
- ^ Shors 2008, p. 564
- ^ Nicholas J (1961). Ross Granville Harrison 1870—1959 A Biographical Memoir (PDF). National Academy of Sciences.
- ^ Steinhardt E, Israeli C, Lambert R (1913). "Studies on the cultivation of the virus of vaccinia". J. Inf Dis. 13 (2): 294–300. doi:10.1093/infdis/13.2.294.
- ^ Maitland HB, Magrath DI (September 1957). "The growth in vitro of vaccinia virus in chick embryo chorio-allantoic membrane, minced embryo and cell suspensions". The Journal of Hygiene. 55 (3): 347–60. doi:10.1017/S0022172400037268. PMC 2217967. PMID 13475780.
- ^ Sussman M, Topley W, Wilson GK, Collier L, Balows A (1998). Topley & Wilson's microbiology and microbial infections. London: Arnold. p. 4. ISBN 978-0-340-66316-5.
- ^ Joklik WK (May 1999). "When two is better than one: thoughts on three decades of interaction between Virology and the Journal of Virology". J. Virol. 73 (5): 3520–3. doi:10.1128/JVI.73.5.3520-3523.1999. PMC 104123. PMID 10196240.
- ^ Schlesinger RW, Granoff A (1994). "George K. Hirst (1909–1994)". Virology. 200 (2): 327. doi:10.1006/viro.1994.1196.
- ^ Shors 2008, pp. 238–344
- ^ Oldstone MB (2009). Viruses, Plagues, and History: Past, Present and Future. Oxford University Press. p. 306. ISBN 978-0-19-532731-1.
- ^ Cunha BA (March 2004). "Influenza: historical aspects of epidemics and pandemics". Infectious Disease Clinics of North America. 18 (1): 141–55. doi:10.1016/S0891-5520(03)00095-3. PMID 15081510.
- ^ Oldstone 2009, p. 315
- ^ Goodpasture EW, Woodruff AM, Buddingh GJ (1931). "The cultivation of vaccine and other viruses in the chorioallantoic membrane of chick embryos". Science. 74 (1919): 371–2. Bibcode:1931Sci....74..371G. doi:10.1126/science.74.1919.371. PMID 17810781.
- ^ Kilbourne ED (November 1975). "Presentation of the Academy Medal to George K. Hirst, M.D". Bull N Y Acad Med. 51 (10): 1133–6. PMC 1749565. PMID 1104014.
- ^ Rosen FS (2004). "Isolation of poliovirus—John Enders and the Nobel Prize". New England Journal of Medicine. 351 (15): 1481–83. doi:10.1056/NEJMp048202. PMID 15470207.
- ^ Magrath I (September 2009). "Lessons from clinical trials in African Burkitt lymphoma". Current Opinion in Oncology. 21 (5): 462–8. doi:10.1097/CCO.0b013e32832f3dcd. PMID 19620863. S2CID 32921007.
- ^ Epstein MA (2005). "1. The origins of EBV research: discovery and characterization of the virus". In Robertson ES (ed.). Epstein-Barr Virus. Trowbridge: Cromwell Press. pp. 1–14. ISBN 978-1-904455-03-5.
- ^ Bornkamm GW (April 2009). "Epstein-Barr virus and the pathogenesis of Burkitt's lymphoma: more questions than answers". International Journal of Cancer. 124 (8): 1745–55. doi:10.1002/ijc.24223. PMID 19165855.
- ^ Thorley-Lawson DA (August 2005). "EBV the prototypical human tumor virus—just how bad is it?". The Journal of Allergy and Clinical Immunology. 116 (2): 251–61, quiz 262. doi:10.1016/j.jaci.2005.05.038. PMID 16083776.
- ^ Norrby E (2008). "Nobel Prizes and the emerging virus concept". Archives of Virology. 153 (6): 1109–23. doi:10.1007/s00705-008-0088-8. PMID 18446425. S2CID 10595263.
- ^ "Discoverers and Discoveries - ICTV Files and Discussions". 11 November 2009. Archived from the original on 11 November 2009. Retrieved 5 November 2017.
- ^ Olafson P, MacCallum AD, Fox FH (July 1946). "An apparently new transmissible disease of cattle". The Cornell Veterinarian. 36: 205–13. PMID 20995890.
- ^ Peterhans E, Bachofen C, Stalder H, Schweizer M (2010). "Cytopathic bovine viral diarrhea viruses (BVDV): emerging pestiviruses doomed to extinction". Veterinary Research. 41 (6): 44. doi:10.1051/vetres/2010016. PMC 2850149. PMID 20197026.
- ^ Bryans JT, Crowe ME, Doll ER, McCollum WH (January 1957). "Isolation of a filterable agent causing arteritis of horses and abortion by mares; its differentiation from the equine abortion (influenza) virus". The Cornell Veterinarian. 47 (1): 3–41. PMID 13397177.
- ^ a b Weller TH (December 1995). "Varicella-zoster virus: History, perspectives, and evolving concerns". Neurology. 45 (12 Suppl 8): S9–10. doi:10.1212/wnl.45.12_suppl_8.s9. PMID 8545033. S2CID 37547957.
- ^ a b c Schmidt AC, Johnson TR, Openshaw PJ, Braciale TJ, Falsey AR, Anderson LJ, Wertz GW, Groothuis JR, Prince GA, Melero JA, Graham BS (November 2004). "Respiratory syncytial virus and other pneumoviruses: a review of the international symposium—RSV 2003". Virus Research. 106 (1): 1–13. doi:10.1016/j.virusres.2004.06.008. PMID 15522442.
- ^ a b Griffin DE, Pan CH (2009). "Measles: Old Vaccines, New Vaccines". Measles. Current Topics in Microbiology and Immunology. Vol. 330. pp. 191–212. doi:10.1007/978-3-540-70617-5_10. ISBN 978-3-540-70616-8. PMID 19203111.
- ^ a b Tyrrell DA (August 1987). "The common cold—my favourite infection. The eighteenth Majority Stephenson memorial lecture". The Journal of General Virology. 68 (8): 2053–61. doi:10.1099/0022-1317-68-8-2053. PMID 3039038.
- ^ Zetterström R (March 2008). "Nobel Prize to Baruch Blumberg for the discovery of the aetiology of hepatitis B". Acta Paediatrica. 97 (3): 384–7. doi:10.1111/j.1651-2227.2008.00669.x. PMID 18298788. S2CID 205858963.
- ^ Temin HM, Baltimore D (1972). "RNA-directed DNA synthesis and RNA tumor viruses". Advances in Virus Research Volume 17. Vol. 17. pp. 129–86. doi:10.1016/S0065-3527(08)60749-6. ISBN 9780120398171. PMID 4348509.
- ^ Broder S (January 2010). "The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic". Antiviral Research. 85 (1): 1–18. doi:10.1016/j.antiviral.2009.10.002. PMC 2815149. PMID 20018391.
- ^ Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L (May 1983). "Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS)". Science. 220 (4599): 868–71. Bibcode:1983Sci...220..868B. doi:10.1126/science.6189183. PMID 6189183.
- ^ Houghton M (November 2009). "The long and winding road leading to the identification of the hepatitis C virus". Journal of Hepatology. 51 (5): 939–48. doi:10.1016/j.jhep.2009.08.004. PMID 19781804.
- ^ Peiris JS, Poon LL (2010). "Detection of SARS Coronavirus". Diagnostic Virology Protocols. Methods in Molecular Biology. Vol. 665. pp. 369–82. doi:10.1007/978-1-60761-817-1_20. ISBN 978-1-60761-816-4. PMC 7121416. PMID 21116811.
- ^ Field H, Young P, Yob JM, Mills J, Hall L, Mackenzie J (April 2001). "The natural history of Hendra and Nipah viruses". Microbes and Infection / Institut Pasteur. 3 (4): 307–14. doi:10.1016/S1286-4579(01)01384-3. PMID 11334748.
- ^ Mahy B (2009). Desk Encyclopedia of Human and Medical Virology. Boston: Academic Press. pp. 583–7. ISBN 978-0-12-375147-8.
- ^ Skern T (September 2010). "100 years poliovirus: from discovery to eradication. A meeting report". Archives of Virology. 155 (9): 1371–81. doi:10.1007/s00705-010-0778-x. PMID 20683737. S2CID 9113713.
- ^ Becsei-Kilborn E (2010). "Scientific discovery and scientific reputation: the reception of Peyton Rous' discovery of the chicken sarcoma virus". Journal of the History of Biology. 43 (1): 111–57. doi:10.1007/s10739-008-9171-y. PMID 20503720. S2CID 23924897.
- ^ Gardner CL, Ryman KD (March 2010). "Yellow fever: a reemerging threat". Clinics in Laboratory Medicine. 30 (1): 237–60. doi:10.1016/j.cll.2010.01.001. PMC 4349381. PMID 20513550.
- ^ a b Zacks MA, Paessler S (January 2010). "Encephalitic alphaviruses". Veterinary Microbiology. 140 (3–4): 281–6. doi:10.1016/j.vetmic.2009.08.023. PMC 2814892. PMID 19775836.
- ^ Johnson CD, Goodpasture EW (January 1934). "An investigation of the etiology of mumps". The Journal of Experimental Medicine. 59 (1): 1–19. doi:10.1084/jem.59.1.1. PMC 2132344. PMID 19870227.
- ^ Misra UK, Kalita J (June 2010). "Overview: Japanese encephalitis". Progress in Neurobiology. 91 (2): 108–20. doi:10.1016/j.pneurobio.2010.01.008. PMID 20132860. S2CID 7517544.
- ^ Ross TM (March 2010). "Dengue virus". Clinics in Laboratory Medicine. 30 (1): 149–60. doi:10.1016/j.cll.2009.10.007. PMC 7115719. PMID 20513545.
- ^ Melnick JL (December 1993). "The discovery of the enteroviruses and the classification of poliovirus among them". Biologicals. 21 (4): 305–9. doi:10.1006/biol.1993.1088. PMID 8024744.
- ^ Martin, Malcolm A., Knipe, David M., Fields, Bernard N., Howley, Peter M., Griffin, Diane, Lamb, Robert (2007). Fields' virology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 2395. ISBN 978-0-7817-6060-7.
- ^ Douglass N, Dumbell K (December 1992). "Independent evolution of monkeypox and variola viruses". Journal of Virology. 66 (12): 7565–7. doi:10.1128/JVI.66.12.7565-7567.1992. PMC 240470. PMID 1331540.
- ^ Cooper LZ (1985). "The history and medical consequences of rubella". Reviews of Infectious Diseases. 7 (Suppl 1): S2–10. doi:10.1093/clinids/7.supplement_1.s2. PMID 3890105.
- ^ Yap SF (June 2004). "Hepatitis B: review of development from the discovery of the "Australia Antigen" to end of the twentieth Century". The Malaysian Journal of Pathology. 26 (1): 1–12. PMID 16190102.
- ^ Epstein MA, Achong BG, Barr YM, Zajac B, Henle G, Henle W (October 1966). "Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji)". Journal of the National Cancer Institute. 37 (4): 547–59. doi:10.1093/jnci/37.4.547. PMID 4288580.
- ^ Karpas A (November 2004). "Human retroviruses in leukaemia and AIDS: reflections on their discovery, biology and epidemiology". Biological Reviews of the Cambridge Philosophical Society. 79 (4): 911–33. doi:10.1017/S1464793104006505. PMID 15682876. S2CID 11865468.
- ^ Curtis N (2006). "Viral haemorrhagic fevers caused by Lassa, Ebola and Marburg viruses". Hot Topics in Infection and Immunity in Children III. Advances in Experimental Medicine and Biology. Vol. 582. pp. 35–44. doi:10.1007/0-387-33026-7_4. ISBN 978-0-387-31783-0. PMID 16802617.
- ^ Hartman AL, Towner JS, Nichol ST (March 2010). "Ebola and marburg hemorrhagic fever". Clinics in Laboratory Medicine. 30 (1): 161–77. doi:10.1016/j.cll.2009.12.001. PMID 20513546.
- ^ Kapikian AZ (May 2000). "The discovery of the 27-nm Norwalk virus: an historic perspective". The Journal of Infectious Diseases. 181 (Suppl 2): S295–302. doi:10.1086/315584. PMC 7110248. PMID 10804141.
- ^ Bishop RF, Cameron DJ, Barnes GL, Holmes IH, Ruck BJ (1976). "The Aetiology of Diarrhoea in Newborn Infants". Ciba Foundation Symposium 42 - Acute Diarrhoea in Childhood. Novartis Foundation Symposia. Vol. 42. pp. 223–36. doi:10.1002/9780470720240.ch13. ISBN 9780470720240. PMID 186236.
{{cite book}}:|journal=ignored (help) - ^ Gust ID, Coulepis AG, Feinstone SM, Locarnini SA, Moritsugu Y, Najera R, Siegl G (1983). "Taxonomic classification of hepatitis A virus". Intervirology. 20 (1): 1–7. doi:10.1159/000149367. PMID 6307916.
- ^ Cossart Y (October 1981). "Parvovirus B19 finds a disease". Lancet. 2 (8253): 988–9. doi:10.1016/S0140-6736(81)91185-5. PMID 6117755. S2CID 13336358.
- ^ Feldmann H, Geisbert TW (November 2010). "Ebola haemorrhagic fever". Lancet. 377 (9768): 849–862. doi:10.1016/S0140-6736(10)60667-8. PMC 3406178. PMID 21084112.
- ^ a b Gallo RC (September 2005). "History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2". Oncogene. 24 (39): 5926–30. doi:10.1038/sj.onc.1208980. PMID 16155599.
- ^ Montagnier L (February 2010). "25 years after HIV discovery: prospects for cure and vaccine". Virology. 397 (2): 248–54. doi:10.1016/j.virol.2009.10.045. PMID 20152474.
- ^ De Bolle L, Naesens L, De Clercq E (January 2005). "Update on human herpesvirus 6 biology, clinical features, and therapy". Clinical Microbiology Reviews. 18 (1): 217–45. doi:10.1128/CMR.18.1.217-245.2005. PMC 544175. PMID 15653828.
- ^ Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M (April 1989). "Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome". Science. 244 (4902): 359–62. Bibcode:1989Sci...244..359C. CiteSeerX 10.1.1.469.3592. doi:10.1126/science.2523562. PMID 2523562.
- ^ Bihl F, Negro F (May 2010). "Hepatitis E virus: a zoonosis adapting to humans". The Journal of Antimicrobial Chemotherapy. 65 (5): 817–21. doi:10.1093/jac/dkq085. PMID 20335188.
- ^ Harding A, Lanese N, Harvey A (14 December 2021). "The deadliest viruses in history". Live Science. Retrieved 23 February 2022.
- ^ Wild TF (March 2009). "Henipaviruses: a new family of emerging Paramyxoviruses". Pathologie-biologie. 57 (2): 188–96. doi:10.1016/j.patbio.2008.04.006. PMID 18511217.
- ^ Okamoto H (2009). "History of Discoveries and Pathogenicity of TT Viruses". TT Viruses. Current Topics in Microbiology and Immunology. Vol. 331. pp. 1–20. doi:10.1007/978-3-540-70972-5_1. ISBN 978-3-540-70971-8. PMID 19230554.


