Search results
Appearance
There is a page named "Atomic orbitals" on Wikipedia
- are generally complex-valued. Real-valued orbitals can be formed as linear combinations of mℓ and −mℓ orbitals, and are often labeled using associated harmonic...84 KB (10,923 words) - 06:42, 19 August 2024
- In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies,...33 KB (3,164 words) - 10:14, 24 April 2024
- whole, so the atomic orbitals combine to form molecular orbitals. The electrons from the constituent atoms occupy the molecular orbitals. Mathematically...35 KB (4,390 words) - 11:31, 6 June 2024
- molecular orbitals. It is assumed that the molecular orbital wave function ψj can be written as a simple weighted sum of the n constituent atomic orbitals χi...22 KB (2,943 words) - 17:32, 23 June 2024
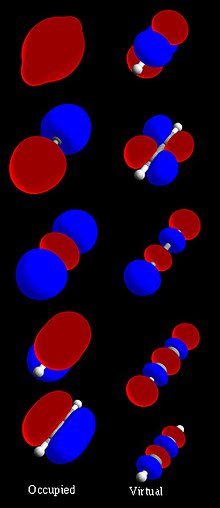
- combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry...6 KB (781 words) - 00:03, 6 April 2023
- Sigma bond (redirect from Sigma orbitals)covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for...8 KB (916 words) - 14:51, 5 August 2024
- Electron configuration (redirect from Atomic electron configuration)electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is...60 KB (6,147 words) - 16:13, 17 August 2024
- Periodic table (redirect from Atomic table)properties. When atomic orbitals overlap during metallic or covalent bonding, they create both bonding and antibonding molecular orbitals of equal capacity...252 KB (27,214 words) - 13:36, 29 August 2024
- Chemical bond (redirect from Atomic bond)which includes orbital hybridization and resonance, and molecular orbital theory which includes the linear combination of atomic orbitals and ligand field...40 KB (4,876 words) - 20:13, 24 May 2024
- Pi bond (redirect from Pi Orbitals)orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals has...7 KB (853 words) - 15:44, 25 August 2024
- are normally higher in energy than bonding molecular orbitals. Bonding and antibonding orbitals form when atoms combine into molecules. If two hydrogen...7 KB (880 words) - 07:57, 10 May 2023
- of atomic orbitals combine to form the same number of molecular orbitals, although the electrons involved may be redistributed among the orbitals. This...38 KB (4,966 words) - 02:30, 13 March 2024
- combination of atomic orbitals. These atomic orbitals are called Slater-type orbitals. Furthermore, it is very common for the "atomic orbitals" in use to...31 KB (4,729 words) - 12:30, 5 August 2024
- Slater-type orbitals (STOs) are functions used as atomic orbitals in the linear combination of atomic orbitals molecular orbital method. They are named...15 KB (2,484 words) - 06:41, 17 November 2023
- orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of...12 KB (1,576 words) - 07:10, 29 April 2024
- between the atoms. This tunneling splits (hybridizes) the atomic orbitals into molecular orbitals with different energies.: 117–122 Similarly, if a large...37 KB (4,835 words) - 14:38, 19 August 2024
- Atom (redirect from Atomic chemical)quantized) set of these orbitals exist around the nucleus, as other possible wave patterns rapidly decay into a more stable form. Orbitals can have one or more...125 KB (12,755 words) - 06:17, 26 August 2024
- have also been proposed in which nucleons occupy orbitals, much like the atomic orbitals in atomic physics theory. These wave models imagine nucleons...34 KB (4,002 words) - 06:55, 14 August 2024
- Azimuthal quantum number (redirect from Orbital quantum number)instance, the n = 1 shell has only orbitals with ℓ = 0 {\displaystyle \ell =0} , and the n = 2 shell has only orbitals with ℓ = 0 {\displaystyle \ell =0}...19 KB (2,121 words) - 15:28, 24 April 2024
- of atomic orbitals can be used: Gaussian-type orbitals, Slater-type orbitals, or numerical atomic orbitals. Out of the three, Gaussian-type orbitals are...36 KB (4,947 words) - 15:58, 6 December 2023
- atomic orbitals plural of atomic orbital
- Lecture: The Atomic Theory (1914) by J. J. Thomson 1154604Romanes Lecture: The Atomic Theory1914J. J. Thomson THE ROMANES LECTURE 1914 The Atomic Theory BY
- function, or orbital, and is denoted by the Greek letter psi (ψ). … What do orbitals look like? There are four different kinds of orbitals, denoted s,
- Combination of Atomic Orbitals (LCAO) is the sum of atomic wave functions that lead to the formation of molecular orbitals. -The symmetry of the orbitals must have