Search results
Appearance
There is a page named "Aminoxyl group" on Wikipedia
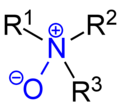
- Aminoxyl denotes a radical functional group with general structure R2N–O•. It is commonly known as a nitroxyl radical or a nitroxide, however IUPAC discourages...3 KB (326 words) - 18:50, 18 February 2023
- methyl groups adjacent to the aminoxyl group. These methyl groups serve as inert substituents, whereas any CH center adjacent to the aminoxyl would be...10 KB (981 words) - 12:58, 26 July 2024
- equal intensity with a spacing of about 13 G (1.3 mT). The inorganic aminoxyl group is a persistent radical, akin to TEMPO. It has been used in some oxidation...11 KB (1,045 words) - 16:21, 17 April 2024
- Amine oxide (category Functional groups)hatchability. Functional group Amine, NR3 Hydroxylamine, NR2OH Phosphine oxide, PR3=O Sulfoxide, R2S=O Azoxy, RN=N+(O−)R RN=N+RO− Aminoxyl group, radicals with...12 KB (1,327 words) - 17:22, 31 July 2024
- oxidation. By these reactions HALS are oxidised to their corresponding aminoxyl radicals (R2NO• c.f. TEMPO), however they are able to return to their initial...5 KB (614 words) - 12:18, 24 September 2023
- labelled with aminoxyl spin label moiety at various carbons (5, 7, 9, 12, 13, 14 and 16) with respect to first carbon of carbonyl group have been used...3 KB (389 words) - 15:53, 2 February 2022
- like HPHA and DEHA, are also thought to react through the intermediary of aminoxyl radicals. Not all inhibitors are radicals however, with quinones and quinone...7 KB (785 words) - 09:28, 26 May 2024
- needed] Nitroxyl radicals (also called aminoxyl radicals) — chemical species containing the R2N−O• functional group "Nitroxyl". PubChem. Retrieved August...9 KB (898 words) - 05:21, 15 April 2024
- subsequently O-ethylhydroxylamine. A variety of functional groups can be oxidized with the aminoxyl radical (phthalimide-N-oxyl, PINO) formed by the abstraction...19 KB (1,927 words) - 16:55, 7 March 2024
- an oxoammonium cation, and at the cathode, the radical is reduced to an aminoxyl anion. These processes are reversed upon discharge, and the radicals are...25 KB (2,964 words) - 18:54, 3 December 2023
- N-Oxoammonium salt (category Functional groups)carbonyls and structurally related to aldoximes (hydroxylamines), and aminoxyl (nitroxide) radicals, with which they can interconvert via a series of...3 KB (280 words) - 17:12, 21 January 2024
- may be explained by the formation of aminoxyl radicals through a process known as the Denisov Cycle. The aminoxyl radical (N-O•) combines with free radicals...27 KB (3,131 words) - 04:54, 12 August 2024
- highest spin nona-carbene ever prepared had a S = 9 ground state (1993). Aminoxyl radicals and pyridylcarbenes were assembled into polymers by coordination...11 KB (1,296 words) - 21:45, 5 June 2024
- that lead to e.g. atom-transfer radical polymerization (ATRP), nitroxide-(aminoxyl) mediated polymerization (NMP), or reversible-addition-fragmentation chain...26 KB (3,025 words) - 08:48, 30 December 2023