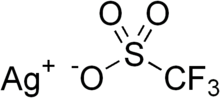Search results
Appearance
There is a page named "AgN3" on Wikipedia
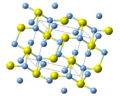
- Silver azide (redirect from AgN3)solution. AgNO3(aq) + NaN3(aq) → AgN3(s) + NaNO3(aq) X-ray crystallography shows that AgN3 is a coordination polymer with square planar Ag+ coordinated...6 KB (453 words) - 15:27, 23 January 2024
- Silver iodide (redirect from AgI)Silver iodide is an inorganic compound with the formula AgI. The compound is a bright yellow solid, but samples almost always contain impurities of metallic...8 KB (610 words) - 20:26, 17 April 2024
- Silver chloride (redirect from AgCl){\displaystyle {\ce {AgNO3 + NaCl -> AgCl(v) + NaNO3}}} 2 AgNO 3 + CoCl 2 ⟶ 2 AgCl ↓ + Co ( NO 3 ) 2 {\displaystyle {\ce {2 AgNO3 + CoCl2 -> 2 AgCl(v) + Co(NO3)2}}}...21 KB (2,249 words) - 10:53, 7 August 2024
- and nitrogen, for example: Pb(N3)2 → Pb + 3 N2 Silver azide AgN3 and barium azide Ba(N3)2 are used similarly. Some organic azides are potential rocket...18 KB (2,060 words) - 10:53, 18 August 2024
- Silver fulminate (redirect from AgONC)Silver fulminate (AgCNO) is the highly explosive silver salt of fulminic acid. Silver fulminate is a primary explosive, but has limited use as such due...9 KB (948 words) - 15:35, 23 January 2024
- such as silver cyanide (AgCN), silver cyanate (AgOCN), silver fulminate (AgCNO), silver thiocyanate (AgSCN) and silver azide (AgN3). A common complex pseudohalide...10 KB (570 words) - 03:27, 28 October 2023
- Tollens' reagent (chemical formula Ag ( NH 3 ) 2 OH {\displaystyle {\ce {Ag(NH3)2OH}}} ) is a chemical reagent used to distinguish between aldehydes and...9 KB (1,092 words) - 15:24, 22 May 2024
- Silver nitrate (redirect from AgNO3)concentration of nitric acid used. 3 Ag + 4 HNO3 (cold and diluted) → 3 AgNO3 + 2 H2O + NO Ag + 2 HNO3 (hot and concentrated) → AgNO3 + H2O + NO2 The structure...22 KB (2,149 words) - 03:10, 31 July 2024
- Silver compounds (redirect from Ag compounds)ethanol. Other dangerously explosive silver compounds are silver azide, AgN3, formed by reaction of silver nitrate with sodium azide, and silver acetylide...18 KB (2,206 words) - 02:26, 1 November 2023
- munitions disposal. Well known detonators are lead azide [Pb(N3)2], silver azide [AgN3] and mercury fulminate [Hg(ONC)2]. There are three categories...30 KB (3,138 words) - 23:47, 21 August 2024
- 30 August 2016. Chung DH, Colon NC, Herndon DN (2012). "Burns". In Coran AG, Caldamone A, Adzick NS, Krummel TM, Laberge J, Shamberger R (eds.). Pediatric...13 KB (1,079 words) - 19:53, 6 August 2024
- Silver bromide (redirect from AgBr)Silver bromide (AgBr) is a soft, pale-yellow, water-insoluble salt well known (along with other silver halides) for its unusual sensitivity to light. This...19 KB (1,941 words) - 06:24, 6 July 2024
- Silver oxide (redirect from AgOH)favorable energetics for the following reaction: 2 AgOH ⟶ Ag 2 O + H 2 O {\displaystyle {\ce {2 AgOH -> Ag2O + H2O}}} (pK = 2.875) With suitably controlled...9 KB (667 words) - 17:46, 23 June 2024
- Silver sulfide (redirect from AgS)Silver sulfide is an inorganic compound with the formula Ag 2S. A dense black solid, it is the only sulfide of silver. It is useful as a photosensitizer...11 KB (970 words) - 10:52, 24 May 2024
- hypochlorite is a chemical compound with the chemical formula AgOCl (also written as AgClO). It is an ionic compound of silver and the polyatomic ion...4 KB (263 words) - 15:55, 13 August 2024
- AgC2H3O2 0.73 0.89 1.05 1.23 1.43 1.93 2.59 Silver azide AgN3 7.931×10−4 Silver bromate AgBrO3 0.11 0.16 0.23 0.32 0.57 0.94 1.33 Silver bromide AgBr...84 KB (193 words) - 10:24, 24 July 2024
- Silver trifluoromethanesulfonate (redirect from AgOTf)trifluoromethanesulfonate, or silver triflate is the triflate (CF3SO3−) salt of Ag+. It is a white or colorless solid that is soluble in water and some organic...5 KB (390 words) - 16:17, 29 September 2023
- respectively, connected to organic/inorganic compounds (e.g. silver azide AgN3, lead azide Pb(N3)2, ammonium azide NH4N3) III. −RnNXm The halogenated nitrogen group...3 KB (423 words) - 07:35, 27 January 2023
- used for plant assays, it is prepared using excess thiosulfate, giving the [Ag(S2O3)2]3– complex ion. "Disilver;dioxido-oxo-sulfanylidene-lambda6-sulfane"...3 KB (184 words) - 16:45, 21 January 2024
- called hydrazoic acid or azoimide (q.v.). It forms salts with metals such as AgN3, which explode in a peculiar manner. The ammonium compound, NH4N3, may become
- If 1.0 g of NaN3 reacts with 25 mL of 0.20 M NaNO3 according to the reaction shown below, how many moles of N2(g) are produced? 5 NaN3(s) + NaNO3(aq)