Gonadotropin-releasing hormone agonist: Difference between revisions
added indication for treatment in high risk paraphilic offenders, with citation. Note Luteinizing Hormone-Releasing Hormone is analogous to GnRH |
Rescuing 2 sources and tagging 0 as dead. #IABot (v1.6.2) (Balon Greyjoy) |
||
| Line 117: | Line 117: | ||
==External links== |
==External links== |
||
* [http://www.patient.co.uk/showdoc/30002442/ Buserelin website] |
* [http://www.patient.co.uk/showdoc/30002442/ Buserelin website] |
||
* [http://www.endometriosis.org/gnrh.html Use of agonists in endometriosis] |
* [https://web.archive.org/web/20051210124110/http://www.endometriosis.org/gnrh.html Use of agonists in endometriosis] |
||
* [http://www.lupron.com/ Lupron, by manufacturer] |
* [http://www.lupron.com/ Lupron, by manufacturer] |
||
* [http://www.kenes.com/gnrh 10th International Symposium on GnRH] |
* [http://www.kenes.com/gnrh 10th International Symposium on GnRH] |
||
* [http://www.supprelinla.com/ SupprelinLA, by Endo Pharmaceuticals, Inc.] |
* [http://www.supprelinla.com/ SupprelinLA, by Endo Pharmaceuticals, Inc.] |
||
* [http://www.zoladex.com/professional/zoladex/ Information of use of Zoladex in prostate cancer] |
* [https://web.archive.org/web/20051208062611/http://www.zoladex.com/professional/zoladex/ Information of use of Zoladex in prostate cancer] |
||
{{Gonadotropins and GnRH}} |
{{Gonadotropins and GnRH}} |
||
Revision as of 16:49, 16 January 2018
| Gonadotropin-releasing hormone agonist | |
|---|---|
| Drug class | |
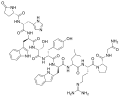
 Leuprorelin, one of the most widely used GnRH agonists. | |
| Class identifiers | |
| Synonyms | GnRH receptor agonists; GnRH blockers; GnRH inhibitors; Antigonadotropins |
| Use | Fertility medicine; Prostate cancer; Breast cancer; Menorrhagia; Endometriosis; Uterine fibroids; Hyperandrogenism; Hirsutism; Precocious puberty; Transgender people; Chemical castration for paraphilias and sex offenders |
| Biological target | GnRH receptor |
| Chemical class | Peptides |
| Legal status | |
| In Wikidata | |
A gonadotropin-releasing hormone agonist (GnRH agonist) is a type of medication which affects gonadotropins and sex hormones.[1] They are used for a variety of indications including in fertility medicine and to lower sex hormone levels in the treatment of hormone-sensitive cancers such as prostate cancer and breast cancer, certain gynecological disorders like heavy periods and endometriosis, high testosterone levels in women, early puberty in children, as a part of transgender hormone therapy, and to delay puberty in transgender youth among other uses. GnRH agonists are given by injections into fat, as implants placed into fat, and as nasal sprays.
Side effects of GnRH agonists are related to sex hormone deficiency and include symptoms of low testosterone levels and low estrogen levels such as hot flashes, sexual dysfunction, vaginal atrophy, osteoporosis, infertility, and diminished sex-specific physical characteristics. They are agonists of the GnRH receptor and work by increasing or decreasing the release of gonadotropins and the production of sex hormones by the gonads. When used to suppress gonadotropin release, GnRH agonists can lower sex hormone levels by 95% in both sexes.[2][3][4][5]
GnRH was discovered in 1971 and GnRH analogues were introduced for medical use in the 1980s.[6][7] The most well-known and widely used GnRH analogue is leuprorelin (brand name Lupron). GnRH analogues are available as generic medications. Despite this however, they continue to be very expensive.
Medical uses
GnRH agonists are useful in:
- Suppression of spontaneous ovulation as part of controlled ovarian hyperstimulation, which is an essential component in in vitro fertilisation (IVF). Typically, after GnRH agonists have induced a state of hypoestrogenism, exogenous FSH is given to stimulate ovarian follicle, followed by human chorionic gonadotropins (hCG) to trigger oocyte release. GnRH agonists routinely used for this purpose are: buserelin, leuprorelin, nafarelin, and triptorelin.[8]
- Final maturation induction after having performed controlled ovarian hyperstimulation. Usage of GnRH agonist for this purpose necessitates using a GnRH antagonist instead of a GnRH agonist for suppression of spontaneous ovulation, because using GnRH agonist for that purpose as well inactivates the axis for which it is intended to work for final maturation induction.
- Treatment of cancers that are hormonally sensitive and where a hypogonadal state decreases the chances of a recurrence. Thus they are commonly employed in the medical management of prostate cancer and have been used in patients with breast cancer.
- Treatment of delaying puberty in individuals with precocious puberty.
- Delaying puberty pending treatment decisions in children with gender dysphoria
- Management of female disorders that are dependent on estrogen productions. Women with menorrhagia, endometriosis, adenomyosis, or uterine fibroids may receive GnRH agonists to suppress ovarian activity and induce a hypoestrogenic state.
- Suppressing sex hormone levels in transgender people, especially transgender women.
- Severe cases of hyperandrogenism, such as in congenital adrenal hyperplasia
- As part of the pharmacologic treatment of paraphilic disorders in sexual offenders or men with a high risk of sexual offending[9]
Women of reproductive age who undergo cytotoxic chemotherapy have been pretreated with GnRH agonists to reduce the risk of oocyte loss during such therapy and preserve ovarian function. Further studies are necessary to prove that this approach is useful.
Available forms
GnRH agonists that have been marketed and are available for medical use include buserelin, gonadorelin, goserelin, histrelin, leuprorelin, nafarelin, and triptorelin. GnRH agonists that are used mostly or exclusively in veterinary medicine include deslorelin and fertirelin. GnRH agonists can be administered by injection, by implant, or intranasally as a nasal spray. Injectables have been formulated for daily, monthly, and quarterly use, and implants are available that can last from one month to a year. With the exception of gonadorelin, which is used as a progonadotropin, all approved GnRH agonists are used as antigonadotropins.
| Overview | ||||
|---|---|---|---|---|
| Name | Brand name(s) | Approved uses | Route(s) | Launch |
| Buserelin | Suprefact | Breast cancer; Endometrial hyperplasia; Endometriosis; Female infertility (assisted reproduction); Prostate cancer; Uterine fibroids | Nasal spray; Injection; Implant | 1984 |
| Deslorelin | Ovuplant; Suprelorin | Veterinary medicine (assisted reproduction; chemical castration) | Implant; Injection | 1994 |
| Fertirelin | Ovalyse | Veterinary medicine (assisted reproduction) | Injection | 1981 |
| Gonadorelin | Factrel; Others | Cryptorchidism; Delayed puberty; Diagnostic agent (pituitary disorders); Hypogonadotropic hypogonadism; Veterinary medicine (assisted reproduction) | Injection; Infusion pump; Nasal spray | 1978 |
| Goserelin | Zoladex | Breast cancer; Endometriosis; Female infertility (assisted reproduction); Prostate cancer; Uterine diseases (endometrial thinning agent); Uterine fibroids; Uterine hemorrhage | Implant | 1989 |
| Histrelin | Vantas; Supprelin LA | Precocious puberty; Prostate cancer | Implant | 1993 |
| Leuprorelin | Lupron; Eligard | Breast cancer; Endometriosis; Menorrhagia; Precocious puberty; Prostate cancer; Uterine fibroids | Injection; Implant | 1985 |
| Nafarelin | Synarel | Precocious puberty; Endometriosis | Nasal spray | 1990 |
| Triptorelin | Decapeptyl | Breast cancer; Endometriosis; Female infertility (assisted reproduction); Paraphilias; Precocious puberty; Prostate cancer; Uterine fibroids | Injection | 1986 |
Contraindications
GnRH agonists are pregnancy category X drugs.
Side effects
Side effects of the GnRH agonists are signs and symptoms of hypoestrogenism, including hot flashes, headaches, and osteoporosis. In patients under long-term therapy, small amounts of estrogens could be given back (“add-back regimen”) to combat such side effects and to prevent bone wastage. Generally, long-term patients, both male and female, tend to undergo annual DEXA scans to appraise bone density.
There is also a report that GnRH agonists used in the treatment of advanced prostate cancer may increase the risk of heart problems by 30%.[10]
Pharmacology
GnRH agonists acts as agonists of the GnRH receptor, the biological target of gonadotropin-releasing hormone (GnRH). These drugs can be both peptides and small-molecules. They are modeled after the hypothalamic neurohormone GnRH, which interacts with the GnRH receptor to elicit its biologic response, the release of the pituitary hormones follicle-stimulating hormone (FSH) and luteinizing hormone (LH). However, after the initial "flare" response, continued stimulation with GnRH agonists desensitizes the pituitary gland (by causing GnRH receptor downregulation) to GnRH. Pituitary desensitization reduces the secretion of LH and FSH and thus induces a state of hypogonadotropic hypogonadal anovulation, sometimes referred to as “pseudomenopause” or “medical oophorectomy.”[1] GnRH agonists are able to completely shutdown gonadal testosterone production and thereby suppress circulating testosterone levels by 95% or into the castrate/female range in men.[5]
Agonists do not quickly dissociate from the GnRH receptor. As a result, initially there is an increase in FSH and LH secretion (so-called "flare effect"). Levels of LH may increase by up to 10-fold.[11] However, after about 10 to 20 days,[11] a profound hypogonadal effect (i.e. decrease in FSH and LH) is achieved through receptor downregulation by internalization of receptors. Generally this induced and reversible hypogonadism is the therapeutic goal. Following cessation of exogenous GnRH agonist it takes 5 to 8 days before normal gonadotropin secretion is completely restored.[12]
Chemistry
GnRH agonists are synthetically modeled after the natural GnRH decapeptide with specific modifications, usually double and single substitutions and typically in position 6 (amino acid substitution), 9 (alkylation) and 10 (deletion). These substitutions inhibit rapid degradation. Agonists with two substitutions include: leuprolide, buserelin, histrelin, goserelin, and deslorelin. The agents nafarelin and triptorelin are agonists with single substitutions at position 6.
Veterinary uses
GnRH analogues are also used in veterinary medicine. Uses include:
- Temporary suppression of fertility in female dogs
- Induction of ovulation in mares
See also
References
- ^ a b Magon N (October 2011). "Gonadotropin releasing hormone agonists: Expanding vistas". Indian Journal of Endocrinology and Metabolism. 15 (4): 261–7. doi:10.4103/2230-8210.85575. PMC 3193774. PMID 22028996.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ R.A.S Hemat (2 March 2003). Andropathy. Urotext. pp. 120–. ISBN 978-1-903737-08-8.
- ^ Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 973–. ISBN 978-0-7817-1750-2.
- ^ S.L. Corson; R.J. Derman (15 December 1995). Fertility Control. CRC Press. pp. 249–250. ISBN 978-0-9697978-0-7.
- ^ a b Novara G, Galfano A, Secco S, Ficarra V, Artibani W (2009). "Impact of surgical and medical castration on serum testosterone level in prostate cancer patients". Urol. Int. 82 (3): 249–55. doi:10.1159/000209352. PMID 19440008.
- ^ David K. Gardner; Carlos Simón (26 June 2017). Handbook of In Vitro Fertilization, Fourth Edition. CRC Press. pp. 131–. ISBN 978-1-4987-2947-5.
- ^ J. Larry Jameson; Leslie J. De Groot (25 February 2015). Endocrinology: Adult and Pediatric E-Book. Elsevier Health Sciences. pp. 2135–. ISBN 978-0-323-32195-2.
- ^ van Loenen AC, Huirne JA, Schats R, Hompes PG, Lambalk CB (November 2002). "GnRH agonists, antagonists, and assisted conception". Seminars in Reproductive Medicine. 20 (4): 349–64. doi:10.1055/s-2002-36713. PMID 12536358.
- ^ Turner, Daniel; Briken, Peer (January 2018). "Treatment of Paraphilic Disorders in Sexual Offenders or Men With a Risk of Sexual Offending With Luteinizing Hormone-Releasing Hormone Agonists: An Updated Systematic Review". The Journal of Sexual Medicine. 15 (1): 77–93. doi:10.1016/j.jsxm.2017.11.013. ISSN 1743-6109. PMID 29289377.
- ^ "Researchers Suggest Hormone Therapy for Prostate Cancer Can Cause Serious Heart Problems and Death". Genetic Engineering & Biotechnology News. 22 September 2009.
- ^ a b Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (25 August 2011). Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. pp. 2939–. ISBN 978-1-4160-6911-9.
- ^ Cedrin-Durnerin, I. (2000). "Consequences on gonadotrophin secretion of an early discontinuation of gonadotrophin-releasing hormone agonist administration in short-term protocol for in-vitro fertilization". Human Reproduction. 15 (5): 1009–1014. doi:10.1093/humrep/15.5.1009. ISSN 1460-2350.