Isomerization: Difference between revisions
m →Instances of isomerization: cap |
Rescuing 1 sources and tagging 0 as dead. #IABot (v1.6.1) |
||
| Line 13: | Line 13: | ||
:[[Image:Rasveratrol isomerization.png|400px|Resveratrol photoisomerization]] |
:[[Image:Rasveratrol isomerization.png|400px|Resveratrol photoisomerization]] |
||
Other instances are [[Aldose-ketose isomerization|aldose-ketose isomerism]] in biochemistry; isomerizations between [[conformational isomer]]s, which take place without an actual rearrangement for instance inconversion of two [[cyclohexane conformation]]s; [[fluxional molecule]]s which display rapid interconversion of isomers e.g. [[bullvalene]]; and "valence isomerization": the isomerization of molecules which involve structural changes resulting only from a relocation of single and [[double bond]]s. If a dynamic equilibrium is established between the two isomers it is also referred to as [[valence tautomerism]].<ref>[http://www.cartage.org.lb/en/themes/Sciences/Chemistry/Organicchemistry/Common/Common.htm Common Definitions and Terms in Organic Chemistry] (from the website of Cartage.org.lb)</ref> |
Other instances are [[Aldose-ketose isomerization|aldose-ketose isomerism]] in biochemistry; isomerizations between [[conformational isomer]]s, which take place without an actual rearrangement for instance inconversion of two [[cyclohexane conformation]]s; [[fluxional molecule]]s which display rapid interconversion of isomers e.g. [[bullvalene]]; and "valence isomerization": the isomerization of molecules which involve structural changes resulting only from a relocation of single and [[double bond]]s. If a dynamic equilibrium is established between the two isomers it is also referred to as [[valence tautomerism]].<ref>[http://www.cartage.org.lb/en/themes/Sciences/Chemistry/Organicchemistry/Common/Common.htm Common Definitions and Terms in Organic Chemistry] {{webarchive|url=https://web.archive.org/web/20100723140400/http://www.cartage.org.lb/en/themes/sciences/chemistry/organicchemistry/common/common.htm |date=2010-07-23 }} (from the website of Cartage.org.lb)</ref> |
||
In a [[cycloisomerization]] a [[cyclic compound]] is formed. Isomerization reactions can also be found with [[Thermal rearrangement of aromatic hydrocarbons|specific aromatic hydrocarbons]]. |
In a [[cycloisomerization]] a [[cyclic compound]] is formed. Isomerization reactions can also be found with [[Thermal rearrangement of aromatic hydrocarbons|specific aromatic hydrocarbons]]. |
||
Revision as of 14:21, 17 November 2017
In chemistry isomerization (also isomerisation) is the process by which one molecule is transformed into another molecule which has exactly the same atoms, but the atoms have a different arrangement e.g. A-B-C → B-A-C (these related molecules are known as isomers [1]). In some molecules and under some conditions, isomerization occurs spontaneously. Many isomers are equal or roughly equal in bond energy, and so exist in roughly equal amounts, provided that they can interconvert somewhat freely; that is, the energy barrier between the two isomers is not too high. When the isomerization occurs intramolecularly it is considered a rearrangement reaction.
An example of an organometallic isomerization is the production of decaphenylferrocene, [(η5-C5Ph5)2Fe] from its linkage isomer.[2][3]
Instances of isomerization
Isomerization in hydrocarbon cracking is usually employed in organic chemistry, where fuels, such as diesel or pentane, a straight-chain isomer, are heated in the presence of a platinum catalyst. The straight- and branched-chain isomers in the resulting mixture then have to be separated. Another industrial process is the isomerisation of n-butane into isobutane.
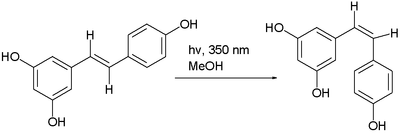
"Trans-cis isomerism" is where, in certain compounds, an interconversion of cis and trans isomers can be observed. For instance, with maleic acid and with azobenzene, often by photoisomerization. Another example is the photochemical conversion of the trans isomer to the cis isomer of resveratrol:[4]
Other instances are aldose-ketose isomerism in biochemistry; isomerizations between conformational isomers, which take place without an actual rearrangement for instance inconversion of two cyclohexane conformations; fluxional molecules which display rapid interconversion of isomers e.g. bullvalene; and "valence isomerization": the isomerization of molecules which involve structural changes resulting only from a relocation of single and double bonds. If a dynamic equilibrium is established between the two isomers it is also referred to as valence tautomerism.[5]
In a cycloisomerization a cyclic compound is formed. Isomerization reactions can also be found with specific aromatic hydrocarbons.
The energy difference between two isomers is called "isomerization energy". Isomerizations with low energy difference both experimental and computational (in parentheses) are endothermic trans-cis isomerization of 2-butene with 2.6 (1.2) kcal/mol, cracking of isopentane to n-pentane with 3.6 (4.0) kcal/mol or conversion of trans-2-butene to 1-butene with 2.6 (2.4) kcal/mol.[6]
See also
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "isomerization". doi:10.1351/goldbook.I03295
- ^ Brown, K. N.; Field, L. D.; Lay, P. A.; Lindall, C. M.; Masters, A. F. (1990). "(η5-Pentaphenylcyclopentadienyl){1-(η6-phenyl)-2,3,4,5-tetraphenylcyclopentadienyl}iron(II), [Fe(η5-C5Ph5){(η6-C6H5)C5Ph4}], a linkage isomer of decaphenylferrocene". J. Chem. Soc., Chem. Commun. (5): 408–410. doi:10.1039/C39900000408.
- ^ Field, L. D.; Hambley, T. W.; Humphrey, P. A.; Lindall, C. M.; Gainsford, G. J.; Masters, A. F.; Stpierre, T. G.; Webb, J. (1995). "Decaphenylferrocene". Aust. J. Chem. 48 (4): 851–860. doi:10.1071/CH9950851.
- ^ Resveratrol Photoisomerization: An Integrative Guided-Inquiry Experiment Elyse Bernard, Philip Britz-McKibbin, Nicholas Gernigon Vol. 84 No. 7 July 2007 Journal of Chemical Education 1159.
- ^ Common Definitions and Terms in Organic Chemistry Archived 2010-07-23 at the Wayback Machine (from the website of Cartage.org.lb)
- ^ How to Compute Isomerization Energies of Organic Molecules with Quantum Chemical Methods Stefan Grimme, Marc Steinmetz, and Martin Korth J. Org. Chem.; 2007; 72(6) pp 2118 - 2126; (Article) doi:10.1021/jo062446p