Essure: Difference between revisions
Bayer with ref - couple of tags applied |
|||
| Line 23: | Line 23: | ||
|medical_notes = |
|medical_notes = |
||
}} |
}} |
||
'''Essure''' is a permanent, non-surgical [[transcervical sterilization]] procedure for women developed by [[Conceptus Inc.]] It was approved for use in the United States on November 4, 2002.<ref name="FDA"/> |
'''Essure''' is a permanent, non-surgical [[transcervical sterilization]] procedure for women developed by [[Conceptus Inc.]], a fully-owned subsidiary of [[Bayer AG]] of [[Germany]] since June 5, 2013.<ref>{{cite web|url=http://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/ucm371014.htm|title=Essure Permanent Birth Control|publisher=U.S. Food and Drug Administration|accessdate=June 24, 2014}}</ref> It was approved for use in the United States on November 4, 2002.<ref name="FDA"/> |
||
Two economic studies, one of which implemented Essure as an in-office procedure, suggest that Essure could be more cost-effective than [[laparoscope|laparoscopic]] bilateral [[tubal ligation]].<ref name=Hurskainen/> |
Two economic studies, one of which implemented Essure as an in-office procedure, suggest that Essure could be more cost-effective than [[laparoscope|laparoscopic]] bilateral [[tubal ligation]].<ref name=Hurskainen/> |
||
==Procedure== |
==Procedure== |
||
The procedure takes about 10 minutes,{{fact|date=June 2014}} for a trained physician to perform and can be performed in a physician's office.{{fact|date=June 2014}} General [[anesthesia]] is not required.<ref>http://www.marketwatch.com/story/conceptusr-to-promote-gynecare-thermachoicer-to-us-physician-offices-2010-11-10?reflink=MW_news_stmp</ref> |
|||
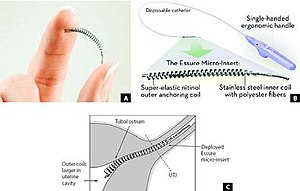
| ⚫ | Small, flexible inserts are placed into the [[fallopian tubes]] by a catheter passed from the [[vagina]] through the [[cervix]] and [[uterus]]. The insert contains inner polyethylene terephthalate fibers to induce benign fibrotic reaction and is held in place by flexible stainless steel inner coil and a dynamic outer nickel titanium alloy coil.<ref>Miño M, Arjona JE, Cordón J, Pelegrin B, Povedano B, Chacon E. Success rate and patient satisfaction with the Essure sterilisation in an outpatient setting: a prospective study of 857 women. BJOG. 2007 Jun;114(6):763-6.</ref> Once in place, the device is designed to elicit tissue growth in and around the insert over a period of three months to form an occlusion or blockage in the fallopian tubes; the tissue barrier formed prevents [[spermatozoon|sperm]] from reaching an egg. |
||
| ⚫ | Small, flexible inserts are placed into the [[fallopian tubes]] by a [[catheter]] passed from the [[vagina]] through the [[cervix]] and [[uterus]].{{fact|date=June 2014}} The insert contains inner polyethylene terephthalate fibers to induce benign fibrotic reaction and is held in place by flexible stainless steel inner coil and a dynamic outer nickel titanium alloy coil.<ref>Miño M, Arjona JE, Cordón J, Pelegrin B, Povedano B, Chacon E. Success rate and patient satisfaction with the Essure sterilisation in an outpatient setting: a prospective study of 857 women. BJOG. 2007 Jun;114(6):763-6.</ref> Once in place, the device is designed to elicit tissue growth in and around the insert over a period of three months to form an occlusion or blockage in the fallopian tubes; the tissue barrier formed prevents [[spermatozoon|sperm]] from reaching an egg.{{fact|date=June 2014}} |
||
Unlike other forms of [[tubal ligation]], no general anaesthetic nor incision through the abdomen is required. |
|||
Similar to some other methods of [[birth control]], initially additional forms of [[birth control]] must be continued for 3 months<ref name=Hurskainen>{{cite doi|10.1016/j.fertnstert.2009.02.080}}</ref> to prevent [[pregnancy]] until the method's effectiveness can be confirmed. |
Unlike other forms of [[tubal ligation]], no general anaesthetic nor incision through the abdomen is required.{{fact|date=June 2014}} Similar to some other methods of [[birth control]], initially additional forms of [[birth control]] must be continued for 3 months<ref name=Hurskainen>{{cite doi|10.1016/j.fertnstert.2009.02.080}}</ref> to prevent [[pregnancy]] until the method's effectiveness can be confirmed. |
||
===Follow-up=== |
===Follow-up=== |
||
For the Essure method, three months after insertion a [[Radiology|Radiologist]] physician performs an x-ray procedure called a [[hysterosalpingogram]]<ref>{{cite web|url=http://www.essure.com/Home/Understanding/EssureConfirmationTest/tabid/187/Default.aspx|title=Essure Confirmation Test|publisher=Conceptus Inc|accessdate=2011-05-30}}</ref> to confirm that the [[fallopian tubes]] are completely blocked and that the patient can rely on the Essure inserts for birth control.<ref name="FDA">{{cite web | title=Essure{{tm}} System - P020014 | url=http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm083087.htm | date=2009-06-29 | publisher=US [[Food and Drug Administration]] | accessdate=2011-05-21}}</ref> A contrast agent (dye) is injected through the cervix, and an x-ray technologist takes photos of the Essure coils to ensure no contrast leaks past the Essure. |
For the Essure method, three months after insertion a [[Radiology|Radiologist]] physician performs an x-ray procedure called a [[hysterosalpingogram]],<ref>{{cite web|url=http://www.essure.com/Home/Understanding/EssureConfirmationTest/tabid/187/Default.aspx|title=Essure Confirmation Test|publisher=Conceptus Inc|accessdate=2011-05-30}}</ref> to confirm that the [[fallopian tubes]] are completely blocked and that the patient can rely on the Essure inserts for birth control.<ref name="FDA">{{cite web | title=Essure{{tm}} System - P020014 | url=http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm083087.htm | date=2009-06-29 | publisher=US [[Food and Drug Administration]] | accessdate=2011-05-21}}</ref> A contrast agent (dye) is injected through the cervix, and an x-ray technologist takes photos of the Essure coils to ensure no contrast leaks past the Essure. |
||
==Efficacy== |
==Efficacy== |
||
| ⚫ | Following successful insertion and occlusional response, the Essure procedure is 99.74% effective based on 5 years of follow-up.<ref>{{cite web | title=Clinical Testing | url=http://essure.com/EssurePermanentBirth control methodsbyConceptus/Understanding/ClinicalTesting/tabid/58/Default.aspx | work=Essure | publisher=Conceptus | accessdate=2006-12-12}} {{Dead link|date=October 2010|bot=H3llBot}}</ref><ref name="pmid19716128">{{cite journal |author=Smith RD |title=Contemporary hysteroscopic methods for female sterilization |journal=Int J Gynaecol Obstet |volume=108 |issue=1 |pages=79–84 |date=January 2010 |pmid=19716128 |doi=10.1016/j.ijgo.2009.07.026 |url=http://linkinghub.elsevier.com/retrieve/pii/S0020-7292(09)00399-3}}</ref> |
||
| ⚫ | Following successful insertion and occlusional response, the Essure procedure is 99.74% effective based on 5 years of follow-up |
||
The reported insertional failure rates are "failure to place 2 inserts in the first procedure (5%), initial tubal patency (3.5%), expulsion (2.2%), perforation (1.8%), or other unsatisfactory device location (0.6%)".<ref name="Precribing"/> Upon follow-up, occlusion is observed to have occurred in 96.5% of patients at 3 months with the remainder occluded by 6 months.<ref name="FDA"/> |
The reported insertional failure rates are "failure to place 2 inserts in the first procedure (5%), initial tubal patency (3.5%), expulsion (2.2%), perforation (1.8%), or other unsatisfactory device location (0.6%)".<ref name="Precribing"/> Upon follow-up, occlusion is observed to have occurred in 96.5% of patients at 3 months with the remainder occluded by 6 months.<ref name="FDA"/> |
||
==Cautions and warnings== |
==Cautions and warnings== |
||
The inserts |
The inserts are made from [[polyester]] fibers, [[nickel]]-[[titanium]] and [[stainless steel]] and [[solder]] and are safe to use with [[MRI]] equipment. Unlike many temporary methods of [[birth control]], the Essure inserts do not contain or release [[hormones]]. The inserts do not prevent the transmission of [[sexually transmitted diseases]]. |
||
| ⚫ | The procedure is reported to be permanent and not reversible by the manufacturing company. Notwithstanding the manufacturer's position, several Essure reversals have been performed.<ref name="reversal">{{cite web | title=Doctors Confirm First Successful Essure Tubal Ligation Reversal|url=http://www.medicalnewstoday.com/articles/125033.php|date=2008-10-08|accessdate=2010-02-15}}, referring to Dr. William A.C. Greene Jr. and Dr. Wendell Turner at Lakeshore Surgical Center</ref> |
||
The inserts are made from [[polyester]] fibers, [[nickel]]-[[titanium]] and [[stainless steel]] and [[solder]] and are safe to use with [[MRI]] equipment.<!-- |
|||
--> |
|||
| ⚫ | The |
||
Additional birth control must be used for three months after procedure.<ref name="Precribing">{{cite web | title=Prescribing Information | url=http://www.essuremd.com/Portals/0/Skins/Conceptus_Skin/PDFs/CC-0366-prescribing-info.pdf | format=PDF | work=Essure | date=2005-09-08 | publisher=Conceptus | accessdate=2006-12-12 |archiveurl = http://web.archive.org/web/20061111074217/http://www.essuremd.com/Portals/0/Skins/Conceptus_Skin/PDFs/CC-0366-prescribing-info.pdf <!-- Bot retrieved archive --> |archivedate = 2006-11-11}}</ref><ref name="pmid19409549">{{cite journal |author=Hurskainen R, Hovi SL, Gissler M, ''et al.'' |title=Hysteroscopic tubal sterilization: a systematic review of the Essure system |journal=Fertil. Steril. |volume= 94|issue= 1|pages= 16–19|date=April 2009 |pmid=19409549 |doi=10.1016/j.fertnstert.2009.02.080 |url=http://linkinghub.elsevier.com/retrieve/pii/S0015-0282(09)00507-X}}</ref> |
Additional birth control must be used for three months after procedure.<ref name="Precribing">{{cite web | title=Prescribing Information | url=http://www.essuremd.com/Portals/0/Skins/Conceptus_Skin/PDFs/CC-0366-prescribing-info.pdf | format=PDF | work=Essure | date=2005-09-08 | publisher=Conceptus | accessdate=2006-12-12 |archiveurl = http://web.archive.org/web/20061111074217/http://www.essuremd.com/Portals/0/Skins/Conceptus_Skin/PDFs/CC-0366-prescribing-info.pdf <!-- Bot retrieved archive --> |archivedate = 2006-11-11}}</ref><ref name="pmid19409549">{{cite journal |author=Hurskainen R, Hovi SL, Gissler M, ''et al.'' |title=Hysteroscopic tubal sterilization: a systematic review of the Essure system |journal=Fertil. Steril. |volume= 94|issue= 1|pages= 16–19|date=April 2009 |pmid=19409549 |doi=10.1016/j.fertnstert.2009.02.080 |url=http://linkinghub.elsevier.com/retrieve/pii/S0015-0282(09)00507-X}}</ref> |
||
| Line 70: | Line 66: | ||
===2013 Controversy=== |
===2013 Controversy=== |
||
In 2013, the product made news with women complaining of severe side effects leading to surgical extraction. According to one article, women who have gotten pregnant are naming these children e-babies. There is a Facebook group of over 6,000 women, as of March 3, 2014, titled Essure Problems. Erin Brockovich also hosts a website where women can share their stories after having the procedure.<ref>[http://articles.chicagotribune.com/2013-12-22/health/ct-essure-safety-met-20131222_1_essure-conceptus-fallopian-tubes Women report complicatidiseasesons from Essure birth control] from [[Chicago Tribune]] retrieved 2 March 2014</ref> |
In 2013, the product made news with women complaining of severe side effects leading to surgical extraction. According to one article, women who have gotten pregnant are naming these children e-babies. There is a Facebook group of over 6,000 women, as of March 3, 2014, titled Essure Problems. [[Erin Brockovich]] also hosts a website where women can share their stories after having the procedure.<ref>[http://articles.chicagotribune.com/2013-12-22/health/ct-essure-safety-met-20131222_1_essure-conceptus-fallopian-tubes Women report complicatidiseasesons from Essure birth control] from [[Chicago Tribune]] retrieved 2 March 2014</ref> |
||
==References== |
==References== |
||
Revision as of 12:50, 24 June 2014
| Essure | |
|---|---|
| Background | |
| Type | Sterilization |
| First use | 2002 |
| Failure rates (first year, after occlusion) | |
| Perfect use | 0.26% |
| Typical use | 0.26% |
| Usage | |
| Duration effect | Permanent |
| Reversibility | Irreversible |
| User reminders | Additional methods until 3 month check by hysterosalpingogram |
| Clinic review | None |
| Advantages and disadvantages | |
| STI protection | No |
| Benefits | Permanent contraception |
Essure is a permanent, non-surgical transcervical sterilization procedure for women developed by Conceptus Inc., a fully-owned subsidiary of Bayer AG of Germany since June 5, 2013.[1] It was approved for use in the United States on November 4, 2002.[2]
Two economic studies, one of which implemented Essure as an in-office procedure, suggest that Essure could be more cost-effective than laparoscopic bilateral tubal ligation.[3]
Procedure
The procedure takes about 10 minutes,[citation needed] for a trained physician to perform and can be performed in a physician's office.[citation needed] General anesthesia is not required.[4]
Small, flexible inserts are placed into the fallopian tubes by a catheter passed from the vagina through the cervix and uterus.[citation needed] The insert contains inner polyethylene terephthalate fibers to induce benign fibrotic reaction and is held in place by flexible stainless steel inner coil and a dynamic outer nickel titanium alloy coil.[5] Once in place, the device is designed to elicit tissue growth in and around the insert over a period of three months to form an occlusion or blockage in the fallopian tubes; the tissue barrier formed prevents sperm from reaching an egg.[citation needed]
Unlike other forms of tubal ligation, no general anaesthetic nor incision through the abdomen is required.[citation needed] Similar to some other methods of birth control, initially additional forms of birth control must be continued for 3 months[3] to prevent pregnancy until the method's effectiveness can be confirmed.
Follow-up
For the Essure method, three months after insertion a Radiologist physician performs an x-ray procedure called a hysterosalpingogram,[6] to confirm that the fallopian tubes are completely blocked and that the patient can rely on the Essure inserts for birth control.[2] A contrast agent (dye) is injected through the cervix, and an x-ray technologist takes photos of the Essure coils to ensure no contrast leaks past the Essure.
Efficacy
Following successful insertion and occlusional response, the Essure procedure is 99.74% effective based on 5 years of follow-up.[7][8]
The reported insertional failure rates are "failure to place 2 inserts in the first procedure (5%), initial tubal patency (3.5%), expulsion (2.2%), perforation (1.8%), or other unsatisfactory device location (0.6%)".[9] Upon follow-up, occlusion is observed to have occurred in 96.5% of patients at 3 months with the remainder occluded by 6 months.[2]
Cautions and warnings
The inserts are made from polyester fibers, nickel-titanium and stainless steel and solder and are safe to use with MRI equipment. Unlike many temporary methods of birth control, the Essure inserts do not contain or release hormones. The inserts do not prevent the transmission of sexually transmitted diseases.
The procedure is reported to be permanent and not reversible by the manufacturing company. Notwithstanding the manufacturer's position, several Essure reversals have been performed.[10]
Additional birth control must be used for three months after procedure.[9][11]
Risks
- Perforation, expulsion, or other unsatisfactory location of the insert
- Pregnancy and increased risk of ectopic pregnancy
- Pain, cramping, vaginal bleeding, menstrual pattern changes, light periods at first then longer, heavier periods lasting up to 6–8 weeks due to changing birth control methods to a non-hormonal solution
- Nausea/vomiting
- Vasovagal response (fainting)
- Allergic reaction to the materials
- Heightened allergic response to other allergens
- heavy metal toxicity
- itchy, raised rash
- brain fog
- autoimmune disease symptoms
- weight gain
- anxiety/depression
- hair loss
2013 Controversy
In 2013, the product made news with women complaining of severe side effects leading to surgical extraction. According to one article, women who have gotten pregnant are naming these children e-babies. There is a Facebook group of over 6,000 women, as of March 3, 2014, titled Essure Problems. Erin Brockovich also hosts a website where women can share their stories after having the procedure.[12]
References
- ^ "Essure Permanent Birth Control". U.S. Food and Drug Administration. Retrieved June 24, 2014.
- ^ a b c "Essure[[Trademark|™]] System - P020014". US Food and Drug Administration. 2009-06-29. Retrieved 2011-05-21.
{{cite web}}: URL–wikilink conflict (help) - ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.fertnstert.2009.02.080, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.fertnstert.2009.02.080instead. - ^ http://www.marketwatch.com/story/conceptusr-to-promote-gynecare-thermachoicer-to-us-physician-offices-2010-11-10?reflink=MW_news_stmp
- ^ Miño M, Arjona JE, Cordón J, Pelegrin B, Povedano B, Chacon E. Success rate and patient satisfaction with the Essure sterilisation in an outpatient setting: a prospective study of 857 women. BJOG. 2007 Jun;114(6):763-6.
- ^ "Essure Confirmation Test". Conceptus Inc. Retrieved 2011-05-30.
- ^ control methodsbyConceptus/Understanding/ClinicalTesting/tabid/58/Default.aspx "Clinical Testing". Essure. Conceptus. Retrieved 2006-12-12.
{{cite web}}: Check|url=value (help) [dead link] - ^ Smith RD (January 2010). "Contemporary hysteroscopic methods for female sterilization". Int J Gynaecol Obstet. 108 (1): 79–84. doi:10.1016/j.ijgo.2009.07.026. PMID 19716128.
- ^ a b "Prescribing Information" (PDF). Essure. Conceptus. 2005-09-08. Archived from the original (PDF) on 2006-11-11. Retrieved 2006-12-12.
- ^ "Doctors Confirm First Successful Essure Tubal Ligation Reversal". 2008-10-08. Retrieved 2010-02-15., referring to Dr. William A.C. Greene Jr. and Dr. Wendell Turner at Lakeshore Surgical Center
- ^ Hurskainen R, Hovi SL, Gissler M; et al. (April 2009). "Hysteroscopic tubal sterilization: a systematic review of the Essure system". Fertil. Steril. 94 (1): 16–19. doi:10.1016/j.fertnstert.2009.02.080. PMID 19409549.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Women report complicatidiseasesons from Essure birth control from Chicago Tribune retrieved 2 March 2014
External links
