Pelargonic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
Nonanoic acid | |
| Other names
Nonoic acid; nonylic acid; 1-octanecarboxylic acid; C9:0 (lipid numbers)
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1752351 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.574 |
| EC Number |
|
| 185341 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H18O2 | |
| Molar mass | 158.241 g/mol |
| Appearance | Clear to yellowish oily liquid |
| Density | 0.900 g/cm3 |
| Melting point | 12.5 °C (54.5 °F; 285.6 K) |
| Boiling point | 254 °C (489 °F; 527 K) |
| Critical point (T, P) | 439 °C (712 K), 2.35 MPa |
| 0.3 g/L | |
| Acidity (pKa) |
|
Refractive index (nD)
|
1.4322 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Corrosive |
| GHS labelling: | |
 
| |
| Warning | |
| H315, H319, H412 | |
| P264, P273, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 114 °C (237 °F; 387 K) |
| 405 °C (761 °F; 678 K) | |
| Related compounds | |
Related compounds
|
Octanoic acid, decanoic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
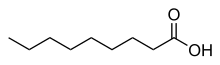
Pelargonic acid, also called nonanoic acid, is an organic compound with structural formula CH3(CH2)7CO2H. It is a nine-carbon fatty acid. Nonanoic acid is a colorless oily liquid with an unpleasant, rancid odor. It is nearly insoluble in water, but very soluble in organic solvents. The esters and salts of pelargonic acid are called pelargonates or nonanoates.
The acid is named after the pelargonium plant, since oil from its leaves contains esters of the acid.
Preparation
Together with azelaic acid, it is produced industrially by ozonolysis of oleic acid.[2]
Alternatively, pelargonic acid can be produced in a two-step process beginning with coupled dimerization and hydroesterification of 1,3-butadiene. This step produces a doubly unsaturated C9-ester, which can be hydrogenated to give esters of pelargonic acid.[3]
A laboratory preparation involves permanganate oxidation of 1-decene.[4]
Occurrence and uses
Pelargonic acid occurs naturally as esters in the oil of pelargonium.
Synthetic esters of pelargonic acid, such as methyl pelargonate, are used as flavorings. Pelargonic acid is also used in the preparation of plasticizers and lacquers. The derivative 4-nonanoylmorpholine is an ingredient in some pepper sprays.
The ammonium salt of pelargonic acid, ammonium pelargonate, is a herbicide. It is commonly used in conjunction with glyphosate, a non-selective herbicide, for a quick burn-down effect in the control of weeds in turfgrass. It works by causing leaks in plant cell membranes, allowing chlorophyll molecules to escape the chloroplast. Under sunlight, these misplaced molecules cause immense oxidative damage to the plant.[5]
The methyl form and ethylene glycol pelargonate act as nematicides against Meloidogyne javanica on Solanum lycopersicum, and the methyl against Heterodera glycines and M. incognita on Glycine max.[6]
Esters of pelargonic acid are precursors to lubricants.
Pharmacological effects
Pelargonic acid may be more potent than valproic acid in treating seizures.[7] Moreover, in contrast to valproic acid, pelargonic acid exhibited no effect on HDAC inhibition, suggesting that it is unlikely to show HDAC inhibition-related teratogenicity.[7]
See also
References
- ^ Lide, D. R. (Ed.) (1990). CRC Handbook of Chemistry and Physics (70th Edn.). Boca Raton (FL):CRC Press.
- ^ David J. Anneken, Sabine Both, Ralf Christoph, Georg Fieg, Udo Steinberner, Alfred Westfechtel "Fatty Acids" in Ullmann's Encyclopedia of Industrial Chemistry, 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a10_245.pub2
- ^ J. Grub; E. Löser (2012). "Butadiene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_431.pub2. ISBN 978-3527306732.
- ^ Lee, Donald G.; Lamb, Shannon E.; Chang, Victor S. (1981). "Carboxylic Acids from the Oxidation of Terminal Alkenes by Permanganate: Nonadecanoic Acid". Organic Syntheses. 60: 11. doi:10.15227/orgsyn.060.0011.
- ^ Lederer, Barbara; Fujimori, Takane; Tsujino, Yasuko; Wakabayashi, Ko; Böger, Peter (November 2004). "Phytotoxic activity of middle-chain fatty acids II: peroxidation and membrane effects". Pesticide Biochemistry and Physiology. 80 (3): 151–156. doi:10.1016/j.pestbp.2004.06.010.
- ^ Chitwood, David J. (2002). "Phytochemical Based Strategies for Nematode Control". Annual Review of Phytopathology. 40 (1). Annual Reviews: 221–249. doi:10.1146/annurev.phyto.40.032602.130045. ISSN 0066-4286. PMID 12147760. p. 229.
- ^ a b Chang, P.; Terbach, N.; Plant, N.; Chen, P. E.; Walker, M. C.; Williams, R. S. (2013). "Seizure control by ketogenic diet-associated medium chain fatty acids". Neuropharmacology. 69: 105–114. doi:10.1016/j.neuropharm.2012.11.004. PMC 3625124. PMID 23177536.




