Ocean surface ecosystem

| Marine habitats |
|---|
| Coastal habitats |
| Ocean surface |
| Open ocean |
| Sea floor |
Organisms that live freely at the ocean surface, termed neuston, include keystone organisms like the golden seaweed Sargassum that makes up the Sargasso Sea, floating barnacles, marine snails, nudibranchs, and cnidarians. Many ecologically and economically important fish species live as or rely upon neuston. Species at the surface are not distributed uniformly; the ocean's surface provides habitat for unique neustonic communities and ecoregions found at only certain latitudes and only in specific ocean basins. But the surface is also on the front line of climate change and pollution. Life on the ocean's surface connects worlds. From shallow waters to the deep sea, the open ocean to rivers and lakes, numerous terrestrial and marine species depend on the surface ecosystem and the organisms found there.[1]
The ocean's surface acts like a skin between the atmosphere above and the water below, and hosts an ecosystem unique to this environment. This sun-drenched habitat can be defined as roughly one metre in depth, as nearly half of UV-B is attenuated within this first meter.[2] Organisms here must contend with wave action and unique chemical [3][4][5] and physical properties.[6] The surface is utilised by a wide range of species, from various fish and cetaceans, to species that ride on ocean debris (termed rafters).[7][8][9]
Most prominently, the surface is home to a unique community of free-living organisms, termed neuston (from the Greek word υεω, which means both to swim and to float). Floating organisms are also sometimes referred to as pleuston, though neuston is more commonly used. Despite the diversity and importance of the ocean's surface in connecting disparate habitats, and the risks it faces, not a lot is known about neustonic life.[1]
Overview
Neuston are key ecological links connecting ecosystems as far ranging as coral reefs, islands, the deep sea, and even freshwater habitats. In the North Pacific, 80% of the loggerhead turtle diet consists of neuston prey,[10] and nearly 30% of the Laysan albatross's diet is neuston.[11] Diverse pelagic and reef fish species live at the surface when young,[12] including commercially important fish species like the Atlantic cod, salmon, and billfish. Neuston can be concentrated as living islands that completely obscure the sea surface, or scattered into sparse meadows over thousands of miles. Yet the role of the neuston, and in many cases their mere existence, is often overlooked.[1]
One of the most well-known surface ecoregions is the Sargasso Sea, an ecologically distinct region packed with thick, neustonic brown seaweed in the North Atlantic. Multiple ecologically and commercially important species depend on the Sargasso Sea, but neustonic life exists in every ocean basin and may serve a similar, if unrecognised, role in regions across the planet. For example, over 50 years ago, USSR scientist A. I. Savilov characterised 7 neustonic ecoregions in the Pacific Ocean.[13] Each ecoregion possesses a unique combination of biotic and abiotic conditions and hosts a unique community of neustonic organisms. Yet these ecoregions have been largely forgotten.[1]
But there is another reason to study neuston: The ocean's surface is on the front line of human impacts, from climate change to pollution, oil spills to plastic. The ocean's surface is hit hard by anthropogenic change, and the surface ecosystem is likely already dramatically different from even a few hundred years ago. For example, prior to widespread damming, logging, and industrialisation, more wood may have entered the open ocean,[14] while plastic had not yet been invented. And because floating life provides food and shelter for diverse species, changes in the surface habitat will cause changes in other ecosystems and have implications that are not currently fully understand or be able to be predicted.[1]
-
Ocean surfaces occupy 72% of the Earth's total surface. They can be divided into surfaces of the relatively shallow and nutrient rich coastal areas above the continental shelves (light blue), and surfaces of the more expansive and relatively deeper but nutrient poor ocean that lies beyond (deep blue).
| External videos | |
|---|---|
"Just before it was dark, as they passed a great island of Sargasso weed that heaved and swung in the light sea as though the ocean were making love with something under a yellow blanket, his small line was taken by a dolphin." — Ernest Hemingway, The Old Man and the Sea.
-
-
Sargasso sea
Ocean surface life (neuston)
Invoking images of the open ocean's surface, the imagination can conjure up an endless empty space. A flat line parting the blue below from the blue above. But in reality a diverse array of species occupy this unique boundary layer. A tangle of terms exist for different organisms occupying different niches of the ocean's surface. The most inclusive term, neuston, is used here to refer to all of them.[1]
Neustonic animals and plants live hanging from the surface of the ocean as if suspended from the roof of a massive cave, and are incapable of controlling their direction of movement. They are considered permanent residents of the surface layer. Many genera are globally distributed. Many organisms have morphological features that enable them to remain at the ocean's surface, with the most noticeable adaptations being floats.[1]
Floaters (pleuston)
| Floaters, sometimes called pleuston, are the organisms that live floating at the ocean surface.[1] | ||
| Cnidarians (jellyfish) |
Velella, Porpita, Physalia, and Actinecta
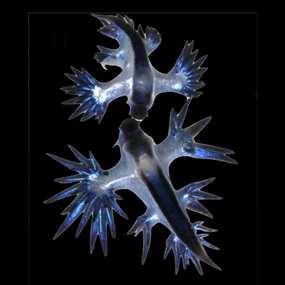
Numerous floating cnidarians (jellyfish) live at the ocean's surface, some famous (or infamous) and others rarely seen. Species like Velella sp. (by-the-wind sailor) and Porpita sp. (blue button) are central to the surface food web. They possess symbiotic dinoflagellates in their tissue, and like their benthic coral cousins, these symbionts may allow them to survive in oligotrophic waters. Velella and Porpita are the only two genera of the chondrophore clade within Hydrozoa, and likely evolved convergently with another neustonic Hydrozoan genera: Physalia (Portuguese man-o-war). Both Physalia and Velella poses "sails", which allow them to travel based on wind direction.[15] These by-the-wind sailors float near the surface of the ocean with their tentacles hanging below in the water. Velella has a raised transparent "sail" on a blue oval disk. Short fringing tentacles hang below from the disc. Movement is powered by wind hitting the sail. Some Velella have a right-hand sail and some a left-hand sail, ensuring they don't all get blown in the one direction at the same time.[16] Physalia also utilises trailing tentacles that serve as a sea anchor on in the open ocean,[17] and pack a powerful sting. Sea anemones in the genus Actinecta are rarely seen, but also float submerged on the ocean's surface, similar to Porpita, but using a bubble float on the pedal disc.[1]
| |
|---|---|---|
| Mollusks (marine snails) |
The mollusks Janthina, Recluzia, and Glaucus
Bubble rafting snails Recluzia and Janthina construct floating rafts by dipping their anterior foot into the water's surface and wrapping trapped air in a layer of mucus to form a bubble, which they then adhere to a raft. The enigmatic Recluzia feeds upon the sea anemone Actinecta, and both are brown-yellow in colour. In contrast, the violet snails Janthina prey on Velella, Porpita, and sometimes Physalia [3], though they cannot move or hunt. Instead, Janthina rely on passive contact with their prey. Other species include the nudibranch Glaucus (blue sea dragon), which also feeds on floating hydrozoans [18] and swallows air to stay afloat. There are multiple cryptic species of Glaucus,[19] and species in this genus may show a high degree of regional isolation.[20][1]
| |
| Crustacean |
The only truly neustonic barnacle, Dosima fascicularis (Buoy barnacle) lives at the ocean's surface by first attaching to floating objects as larvae (such as feathers), and secreting an airy pillow-like float rather than the normal hard cement used by other barnacles.[21][22] This float allows Dosima barnacles to eventually outgrow their larval home and drift independently.[1] | |
| Macroalgae (seaweed) |
Neustonic seaweeds like Sargassum fluitans and Sargassum natans have numerous gas-filled floats to remain at the ocean's surface. These algae create habitat for a variety of Sargassum-associated species, particularly at the western edge of the North Atlantic Subtropical Gyre, known as the Sargasso Sea.[23] In the Pacific the algal genus Turbinaria reproduces with floating fronts.[24] In addition, over 20 species of algal have been found floating at the surface, and eight species of sea grass.[7][1] | |
| Microorganisms (bacteria, protists etc) |
 Trichodesmium bloom Diverse microorganisms occupy the ocean's surface ecosystem,[25][26][27][28][10] and may play a significant role in gas exchange between the ocean and atmosphere.[29] Many of these organisms have been identified from the sea surface microlayer, which, depending on definition, extends from 100-1000 μm below the ocean's surface.[25] The ocean's surface has unique chemical and physical properties that may concentrate species specifically adapted to these conditions. For example, bacterioneuston living in the sea surface microlayer are often brightly coloured,[30] possibly as protection against solar radiation. The surface microlayer may be largely dominated by heterotrophic organisms, including both bacteria and microeukaryotes, which take advantage of surface associated compounds.[27] Other species may extend beyond the sea-surface microlayer but still associated with the surface, including the ecologically important cyanobacterium Trichodesmium.[31] Still, as with larger organisms, surface microorganisms are generally poorly known.[10][1] | |
Epineuston
| Epineuston are organisms that use water surface tension to keep them on the ocean surface.[1] | ||
| Insects |
 Aggregation of sea skaters [32] There are very few marine insects.[33] The only true open-ocean insects are Halobates. Epineustonic organisms live on the water's surface, and in the open ocean all epineustonic species belong to the insect genus Halobates. Known as "sea skaters", Halobates sp. prey on other neustonic species and zooplankton trapped at the surface.[34] Halobates lay eggs on a variety of objects, including floating feathers, wood, plastic etc.,[35] and unusually on pelagic molluscs like Atlanta turriculata.[34][1] | |
|---|---|---|
Hyponeuston
| Hyponeuston are the mobile organisms that live immediately below the surface.[1] | ||
| Copepods |
 A wide variety of copepods can be found at the ocean's surface.[36][37][38][39][40][41][42] Some neustonic copepods possess remarkable adaptations, especially within the pontellid copepods. Pontellid adaptations include specialised surface attachment structures,[43] blue pigmentation.[36][40] and even flying behavior to escape predators.[41] Sapphirinidae copepods are often also associated with the surface,[37] and some species have incredible structural colouration.[42] As in many marine ecosystems, copepods represent a major food source for a variety of neustonic and surface-associated species.[1] The sea surface microlayer (SML) at the air-sea interface is a distinct, under-studied habitat compared to the subsurface and copepods, important components of ocean food webs, have developed key adaptations to exploit this niche.[40] The ocean-spanning SML forms the boundary between the atmosphere and the hydrosphere. Despite having a thickness of less than one millimetre, the SML has profoundly different physicochemical and biological characteristics from the underlying water (ULW).[44] The SML provides a biogenic gelatinous framework [4] and is typically enriched with organic matter,[45] heterotrophic microorganisms [26] as well as higher trophic level organisms.[46][40] Among zooplankton taxa living within the SML, neustonic copepods (phylum Arthropoda, class Crustacea) of the family Pontellidae have been frequently recorded in tropical regions of all oceans.[47][48][49] The SML is regarded as a challenging or even extreme habitat because organisms are exposed to variable temperatures and high intensities of solar and ultraviolet (UV) radiation.[50] Copepods are the most abundant metazoans on Earth [51] and show impressive short-term adaptation to environmental stressors, e.g. downregulation of the cellular heat stress response.[52] Given their major role in marine food webs and ecosystem functioning,[53] knowledge of the tolerance limits of copepods to abiotic factors is essential if robust projections of the effects of global change on the world's oceans are to be possible. The effects of climate-driven warming (and acidification) on the SML ecosystem and neuston-dwelling copepods, although scarcely examined to date, may be particularly dramatic.[40] A feature of many pontellid copepods is their blue colouring, that also occurs in other surface-dwelling mesozooplankton.[54] The colouring results from a complex of the pigment astaxanthin and a carotenoprotein.[55] Astaxanthin can be produced from dietary sources and was found to be the principal carotenoid in four different blue-pigmented copepod genera as well as in Oikopleura dioica of the class Appendicularia indicating convergent evolution of the feature in different neuston inhabitants.[56] Various theories have been developed to explain the significance of the blue colouring in copepods, including protection from strong solar and/or UV radiation,[57][58] camouflage against visual predators that forage in the uppermost water layers [54] as well as recognition of conspecifics when occurring together with copepods that possess a green fluorescent protein (GFP)-based coloration.[59][40] | |
|---|---|---|
| Isopods |
 The isopod Idotea metallica Idotea metallica is a remarkable surface-associated isopod, that can be found either floating upside down on the ocean's surface [1] or attached to floating debris or neuston (such as the bubble rafts of Janthina). It is commonly associated with flotsam,[60] and is capable of actively swimming from one floating object to another. This species ostensibly occurs globally in warm waters, though as with many surface-associated species, information on its genetic diversity is scarce. It is often flushed into more northern regions by shifting currents. For individuals arriving in the summer months in Helgoland (Germany; North Sea) the fundamental thermal niche is 16 °C, with the coldest tolerable temperature likely around 13 °C.[61] However, these thermal tolerance limits should be considered with caution: like many neustonic species, I. metallica is poorly studied, and whether it is truly one species or many cryptic species is unclear.[1] | |
| Shrimp |
Several species of shrimp are associated with floating Sargassum, and may also be found swimming at the surface, including Latreutes fucorum and Hippolyte coerulescens. Neustonic shrimp exhibit a remarkable array of colour patterns,[62] including the common neustonic blue, with chromophores that can respond to changing light conditions.[63][1] | |
| Fish |
A remarkable diversity of fish spend their early life at the ocean's surface. This list includes many well-known, ecologically, and economically important species from a variety of habitats. Pelagic fish species include some anchovy, mahi-mahi, marlins, swordfish, amberjack and Atlantic mackerel. Well-known and ecologically important benthic fish associate with the surface when young, including species of: lefteye flounder, blenny, goby, seahorses, seadragons and pipefish. Deep-sea fish with surface larvae include viperfish and lanternfish. Many eels, both reef, benthic, and deep-sea, nocturnally migrate to the surface layer as larvae.[64] But while the ocean's surface may seem like an odd habitat for larval deep-sea fish, they are far from the most unusual. Diverse fish that migrate into freshwater as adults (either as a habitat or spawning ground) rely on the neuston when young. Yearling and sub-yearling salmon of various species consume neustonic prey in the northern California Current.[65] American and European eels swim from their freshwater rivers and converge in the middle of the North Atlantic to spawn in the Sargasso Sea. Some fish occupy the ocean's surface for their whole lives, and are even capable of soaring above the waves, including flying fish and halfbeaks. Others frequent the ocean's surface, including basking species like sunfish and basking sharks.[1] | |
| Cephalopods |
While no cephalopod is confined to the surface layer permanently, some frequent the surface habitat and are adapted to utilise it. Female argonaut octopus (Argonauta spp.) dip their paper-like shell into the air, trapping gas bubbles that they then use to maintain buoyancy.[66][67] Diverse flying squid species in the Ommastrephidae and Onychoteuthidae can launch themselves from the water and soar for impressive distances, some can reach highs of over three metres and others can sail for distances up to 55 metres.[68] | |
Rafting organisms
| Rafting species live either attached to neustonic organisms (e.g. barnacles that settle on Janthina shells) or inanimate debris. | ||
| barnacles (encrusters) |
Rafting species live either attached to neustonic organisms (e.g. barnacles that settle on Janthina shells) or inanimate debris. Some rafting species have evolved to live on debris at the ocean's surface, such as the smooth gooseneck barnacle Lepas anatifera, while others may be coastal species that settle on near-shore floating debris and are then transported by currents to the open ocean. Several excellent reviews cover the biology of rafters, including the floating substrata of rafters,[7] the rafting community,[8] and the biogeographical and evolutionary consequences of rafting.[9][1] | |
|---|---|---|
Surface microlayer

(II) Distinct microbial communities processing dissolved and particulate organic matter [71]
(III) Highest exposure of solar radiation drives photochemical reactions and formation of radicals [72]
The sea surface microlayer (SML) is the boundary interface between the atmosphere and ocean, covering about 70% of the Earth's surface. With an operationally defined thickness between 1 and 1000 μm, the SML has physicochemical and biological properties that are measurably distinct from underlying waters. Recent studies now indicate that the SML covers the ocean to a significant extent, and evidence shows that it is an aggregate-enriched biofilm environment with distinct microbial communities. Because of its unique position at the air-sea interface, the SML is central to a range of global biogeochemical and climate-related processes.[69]
The sea surface microlayer (SML) is the boundary interface between the atmosphere and ocean, covering about 70% of the Earth's surface. The SML has physicochemical and biological properties that are measurably distinct from underlying waters. Because of its unique position at the air-sea interface, the SML is central to a range of global biogeochemical and climate-related processes. Although known for the last six decades, the SML often has remained in a distinct research niche, primarily as it was not thought to exist under typical oceanic conditions. Recent studies now indicate that the SML covers the ocean to a significant extent,[73] highlighting its global relevance as the boundary layer linking two major components of the Earth system – the ocean and the atmosphere.[69]

In 1983, Sieburth hypothesised that the SML was a hydrated gel-like layer formed by a complex mixture of carbohydrates, proteins, and lipids.[71] In recent years, his hypothesis has been confirmed, and scientific evidence indicates that the SML is an aggregate-enriched biofilm environment with distinct microbial communities.[74] In 1999 Ellison et al. estimated that 200 Tg C yr−1 accumulates in the SML, similar to sedimentation rates of carbon to the ocean's seabed, though the accumulated carbon in the SML probably has a very short residence time.[75] Although the total volume of the microlayer is very small compared to the ocean's volume, Carlson suggested in his seminal 1993 paper that unique interfacial reactions may occur in the SML that may not occur in the underlying water or at a much slower rate there.[70] He therefore hypothesised that the SML plays an important role in the diagenesis of carbon in the upper ocean.[70] Biofilm-like properties and highest possible exposure to solar radiation leads to an intuitive assumption that the SML is a biochemical microreactor.[76][69]
Historically, the SML has been summarized as being a microhabitat composed of several layers distinguished by their ecological, chemical and physical properties with an operational total thickness of between 1 and 1000 μm. In 2005 Hunter defined the SML as a "microscopic portion of the surface ocean which is in contact with the atmosphere and which may have physical, chemical or biological properties that are measurably different from those of adjacent sub-surface waters".[77] He avoids a definite range of thickness as it depends strongly on the feature of interest. A thickness of 60 μm has been measured based on sudden changes of the pH,[78] and could be meaningfully used for studying the physicochemical properties of the SML. At such thickness, the SML represents a laminar layer, free of turbulence, and greatly affecting the exchange of gases between the ocean and atmosphere. As a habitat for neuston (surface-dwelling organisms ranging from bacteria to larger siphonophores), the thickness of the SML in some ways depends on the organism or ecological feature of interest. In 2005, Zaitsev described the SML and associated near-surface layer (down to 5 cm) as an incubator or nursery for eggs and larvae for a wide range of aquatic organisms.[37][69]
Hunter's definition includes all interlinked layers from the laminar layer to the nursery without explicit reference to defined depths.[79] In 2017, Wurl er al. proposed Hunter's definition be validated with a redeveloped SML paradigm that includes its global presence, biofilm-like properties and role as a nursery. The new paradigm pushes the SML into a new and wider context relevant to many ocean and climate sciences.[69]
According to Wurl et al.m the SML can never be devoid of organics due to the abundance of surface-active substances (e.g., surfactants) in the upper ocean [73] and the phenomenon of surface tension at air-liquid interfaces.[80] The SML is analogous to the thermal boundary layer, and remote sensing of the sea surface temperature shows ubiquitous anomalies between the sea surface skin and bulk temperature.[81] Even so the differences in both are driven by different processes. Enrichment, defined as concentration ratios of an analyte in the SML to the underlying bulk water, has been used for decades as evidence for the existence of the SML. Consequently, depletions of organics in the SML are debatable; however, the question of enrichment or depletion is likely to be a function of the thickness of the SML (which varies with sea state;[82] including losses via sea spray, the concentrations of organics in the bulk water,[73] and the limitations of sampling techniques to collect thin layers .[83] Enrichment of surfactants, and changes in the sea surface temperature and salinity, serve as universal indicators for the presence of the SML. Organisms are perhaps less suitable as indicators of the SML because they can actively avoid the SML and/or the harsh conditions in the SML may reduce their populations. However, the thickness of the SML remains "operational" in field experiments because the thickness of the collected layer is governed by the sampling method. Advances in SML sampling technology are needed to improve our understanding of how the SML influences air-sea interactions.[69]
Surface slicks

Slicks are meandering lines of smooth water on the ocean surface that are ubiquitous coastal features around the world.[85] A variety of mechanisms can cause slick formation, including tidal and headland fronts, and as a consequence of subsurface waves called internal waves.[86] Internal wave slicks are generated when internal waves interact with steep seafloor topography and drive areas of convergence and divergence at the ocean surface.[87] The build-up of organic material (surfactants) at the surface modifies surface tension causing a smooth, oil slick-like appearance.[88] The convergent flow can accumulate dense aggregations of plankton including larval fish and invertebrates at or below the ocean surface.[89][90][91][92][93][94][95][96]
Surface slicks are the focal point for numerous trophic and larval connections that are foundational for marine ecosystem function.[96] Life for many marine organisms begins near the ocean surface. Buoyant eggs hatch into planktonic larvae that develop and disperse in the ocean for weeks to months before transitioning into juveniles and eventually finding suitable adult habitat.[97] The pelagic larval stage connects populations and serves as a source of new adults. Oceanic processes affecting the fate of larvae have profound impacts on population replenishment, connectivity, and ecosystem structure.[98] Although it is an important life stage, there is, as of 2021, limited knowledge of the ecology and behaviour of larvae.[96] Understanding the biophysical interactions that govern larval fish survival and transport is essential for predicting and managing marine ecosystems, as well as the fisheries they support.[99][100][96]

The diagram shows: (1) Larval and juvenile stages of fishes from many ocean habitats aggregate in slicks in order to capitalize on dense concentrations of prey (2, phytoplankton, 3, zooplankton, 4, larval invertebrates, 5, eggs, and 6, insects). The increased predator–prey overlap in slicks increases energy flow that propagates up the food-web (dotted blue lines show trophic links), enhancing energy available to higher trophic level predators (icons outlined in blue) including humans. More than 100 species of fishes develop and grow in surface slick nurseries before transitioning to adults (solid white lines radiating outward) in Coral Reefs (7–12), Epipelagic (13–15), and Deep-water (16–17) ocean habitats. As adults these taxa (icons outlined in white) play important ecological functions and provide fisheries resources to local human populations. For example, coastal schooling fishes (7, mackerel scad) are important food and bait fish for humans. Planktivorous fish (8, some damselfishes and triggerfishes) transfer energy from zooplankton up to reef predators like jacks (9),[101] which provide top-down control of reefs [102] and are important targets for shoreline recreational fisherfolk.[103] Grazers (10, chubs) help keep coral reefs from being overgrown by macroalgae.[104] Cryptobenthic fishes such as blennies (11) and benthic macrocrustaceans (12, shrimp, stomatopods, crabs) comprise most of the consumed biomass on reefs.[105][106] In the pelagic ocean, flyingfishes (13) channel energy and nutrients from zooplankton to pelagic predators such as mahi-mahi (14) and billfish (15), both of which utilize slicks as nursery habitat. Larvae of mesopelagic fishes like lanternfish (16) and bathydemersal tripod fishes (17) utilize these surface hotspots before descending to deep-water adult habitat.[96]
The distribution of prey and predators in the ocean is patchy.[107][108] Larval survival depends on prey availability, predation, and transport to suitable habitat, all of which are influenced by ocean conditions.[109] Ocean processes that drive convergent flow such as fronts, internal waves, and eddies, can structure plankton, enhance overlap of predators and prey, and influence larval dispersal.[89][110][111][112][113][114][115][116] Convergent features can also lead to a cascade of effects that ultimately drive food web structure and increase ecosystem productivity.[117][96]
Life history

Life histories connect disparate ecosystems; species that live at the surface during one life history stage may occupy the deep sea, benthos, reefs, or freshwater ecosystems during another. A diversity of fish species utilize the ocean's surface,[119] either as adults or as nursery habitat for eggs and young. In contrast, species floating on the ocean's surface during one life cycle stage often (though not always) have pelagic larval stages. Velella and Porpita release jellyfish (medusae),[120] and while little is known about Porpita medusae, Velella medusae could possibly sink into deeper water,[120] or remain near the surface, where they derive nutrients from zooxanthellae.[118] Janthina have pelagic veliger larvae,[121] and Physalia may release reproductive clusters that drift in the water column. Halobates lay eggs on a variety of objects, including floating objects [34] and pelagic snail shells.[122][1]
All species with pelagic stages must eventually find their way back to the surface. For Velella and Porpita, larvae generated by sexual reproduction of medusae develop small floats, which carry them to the surface.[123][124] For the larvae of Janthina, the transition to surface life includes the degradation of their eyes and vestibule system, and at the same time, the production of an external structure, which has been reported as either a small parachute made of mucus, or a cluster of bubbles, which they ride to the surface.[125][126] Young Halobates may hatch either above or below the surface, and for those below, the surface tension proves a formidable barrier. It may take Halobates nymphs several hours to break through the surface film.[34] Despite the challenges of reaching the surface, there may be benefits to a temporary pelagic life.[1]


(d) these deep-water habitats may allow them to take advantage of counter currents for transport in the direction opposite surface currents (a hypothesis proposed for Velella)[129]
Connectivity of ocean surface ecosystems may be facilitated by the life history of species living there. One hypothesis is that species have pelagic stages to "escape" surface sink regions and repopulate surface source regions, where one life cycle stage drifts on surface currents in one direction, and a pelagic stage either remains geographically localised [130] or drifts in the opposite direction.[131] However, some surface species, such as the endemic species of the Sargasso Sea, may remain geographically isolated throughout their life history. While these hypotheses are intriguing, it is not known if or how life history shapes population/species distribution for most neustonic species. Understanding how life history varies by species is a critical component of assessing both connectivity and conservation of neustonic ecosystems.[1]
Sea spray

A stream of airborne microorganisms circles the planet above weather systems but below commercial air lanes.[132] Some peripatetic microorganisms are swept up from terrestrial dust storms, but most originate from marine microorganisms in sea spray. In 2018, scientists reported that hundreds of millions of viruses and tens of millions of bacteria are deposited daily on every square meter around the planet.[133][134]
These airborne microorganisms form part of the aeroplankton. The aeroplankton are tiny lifeforms that float and drift in the air, carried by the current of the wind; they are the atmospheric analogue to oceanic plankton. Most of the living things that make up aeroplankton are very small to microscopic in size, and many can be difficult to identify because of their tiny size. Scientists collect them for study in traps and sweep nets from aircraft, kites or balloons.[135]
The environmental role of airborne cyanobacteria and microalgae is only partly understood. While present in the air, cyanobacteria and microalgae can contribute to ice nucleation and cloud droplet formation. Cyanobacteria and microalgae can also impact human health.[136][137][138][139][140][141] Depending on their size, airborne cyanobacteria and microalgae can be inhaled by humans and settle in different parts of the respiratory system, leading to the formation or intensification of numerous diseases and ailments, e.g., allergies, dermatitis, and rhinitis.[138][142][143][144]
See also
- Marine larval ecology
- Ocean surface topography
- Surface layer
- Sea spray
- Sea air
- Surface Ocean Lower Atmosphere Study
- Joint Global Ocean Flux Study
- Regional Ocean Modeling System
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac Helm, Rebecca R. (28 April 2021). "The mysterious ecosystem at the ocean's surface". PLOS Biology. 19 (4). Public Library of Science (PLoS): e3001046. doi:10.1371/journal.pbio.3001046. ISSN 1545-7885. PMC 8081451. PMID 33909611.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Fleischmann, Esther M. (1989). "The measurement and penetration of ultraviolet radiation into tropical marine water". Limnology and Oceanography. 34 (8): 1623–1629. Bibcode:1989LimOc..34.1623F. doi:10.4319/lo.1989.34.8.1623. S2CID 86478743.
- ^ Hardy, J.T. (1982). "The sea surface microlayer: Biology, chemistry and anthropogenic enrichment". Progress in Oceanography. 11 (4): 307–328. Bibcode:1982PrOce..11..307H. doi:10.1016/0079-6611(82)90001-5.
- ^ a b Wurl, Oliver; Holmes, Michael (2008). "The gelatinous nature of the sea-surface microlayer". Marine Chemistry. 110 (1–2): 89–97. Bibcode:2008MarCh.110...89W. doi:10.1016/j.marchem.2008.02.009.
- ^ Cunliffe, Michael; Murrell, J Colin (2009). "The sea-surface microlayer is a gelatinous biofilm". The ISME Journal. 3 (9): 1001–1003. doi:10.1038/ismej.2009.69. PMID 19554040. S2CID 32923256.
- ^ Wurl, Oliver; Ekau, Werner; Landing, William M.; Zappa, Christopher J. (2017). "Sea surface microlayer in a changing ocean – A perspective". Elementa: Science of the Anthropocene. 5. doi:10.1525/elementa.228.
- ^ a b c Thiel, M.; Gutow, L. (2005). "I. The floating substrata". In Gibson, Robin (ed.). Oceanography and marine biology : an annual review. Boca Raton, Fla: CRC Press. ISBN 978-0-203-50781-0.
- ^ a b Thiel, M.; Gutow, L. (2005). "II. The rafting organisms and community". In Gibson, Robin (ed.). Oceanography and marine biology : an annual review. Boca Raton, Fla: CRC Press. ISBN 978-0-203-50781-0.
- ^ a b Thiel, M.; Gutow, L. (2005). "III. Biogeographical and evolutionary consequences". In Gibson, Robin (ed.). Oceanography and marine biology : an annual review. Boca Raton, Fla: CRC Press. ISBN 978-0-203-50781-0.
- ^ a b c Rahlff, Janina (2019). "The Virioneuston: A Review on Viral–Bacterial Associations at Air–Water Interfaces". Viruses. 11 (2): 191. doi:10.3390/v11020191. PMC 6410083. PMID 30813345.
- ^ "Hawaiian Seabird Feeding Ecology", Wildlife Monographs, 85: 3-71. Wiley.
- ^ Gove, Jamison M.; Whitney, Jonathan L.; McManus, Margaret A.; Lecky, Joey; Carvalho, Felipe C.; Lynch, Jennifer M.; Li, Jiwei; Neubauer, Philipp; Smith, Katharine A.; Phipps, Jana E.; Kobayashi, Donald R.; Balagso, Karla B.; Contreras, Emily A.; Manuel, Mark E.; Merrifield, Mark A.; Polovina, Jeffrey J.; Asner, Gregory P.; Maynard, Jeffrey A.; Williams, Gareth J. (2019). "Prey-size plastics are invading larval fish nurseries". Proceedings of the National Academy of Sciences. 116 (48): 24143–24149. Bibcode:2019PNAS..11624143G. doi:10.1073/pnas.1907496116. PMC 6883795. PMID 31712423.
- ^ Savilov, A.I. (1969) "Pleuston of the Pacific Ocean". In Zenkewich, LA (Ed.) Biology of the Pacific Ocean: Part 2 The deep sea bottom fauna.
- ^ Lee, Hyejung; Galy, Valier; Feng, Xiaojuan; Ponton, Camilo; Galy, Albert; France-Lanord, Christian; Feakins, Sarah J. (2019). "Sustained wood burial in the Bengal Fan over the last 19 My". Proceedings of the National Academy of Sciences. 116 (45): 22518–22525. Bibcode:2019PNAS..11622518L. doi:10.1073/pnas.1913714116. PMC 6842586. PMID 31636189.
- ^ Ferrer, Luis; Pastor, Ane (2017). "The Portuguese man-of-war: Gone with the wind". Regional Studies in Marine Science. 14: 53–62. Bibcode:2017RSMS...14...53F. doi:10.1016/j.rsma.2017.05.004.
- ^ Browne, J. (2019) Velella velella: By-the-wind Sailor in Museums Victoria Collections, Australia. Accessed 4 December 2021.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Iosilevskii, G.; Weihs, D. (2009). "Hydrodynamics of sailing of the Portuguese man-of-war Physalia physalis". Journal of the Royal Society Interface. 6 (36): 613–626. doi:10.1098/rsif.2008.0457. PMC 2696138. PMID 19091687.
- ^ Bieri, Robert (1966). "Feeding Preferences and Rates of the Snail, Ianthina Prolongata, the Barnacle, Lepas Anserifera, the Nudibranchs, Glaucus Atlanticus and Fiona Pinnata, and the Food Web in the Marine Neuston". Publications of the Seto Marine Biological Laboratory. 14 (2): 161–170. doi:10.5134/175429.
- ^ Churchill, Celia K. C.; Valdés, Ángel; ó Foighil, Diarmaid (2014). "Molecular and morphological systematics of neustonic nudibranchs (Mollusca : Gastropoda : Glaucidae : Glaucus), with descriptions of three new cryptic species". Invertebrate Systematics. 28 (2): 174. doi:10.1071/IS13038. S2CID 84010907.
- ^ Churchill, Celia K. C.; Valdés, Ángel; ó Foighil, Diarmaid (2014). "Afro-Eurasia and the Americas present barriers to gene flow for the cosmopolitan neustonic nudibranch Glaucus atlanticus". Marine Biology. 161 (4): 899–910. doi:10.1007/s00227-014-2389-7. S2CID 84153330.
- ^ Zheden, Vanessa; Kovalev, Alexander; Gorb, Stanislav N.; Klepal, Waltraud (2015). "Characterization of cement float buoyancy in the stalked barnacle Dosima fascicularis (Crustacea, Cirripedia)". Interface Focus. 5 (1). doi:10.1098/rsfs.2014.0060. PMC 4275874. PMID 25657839.
- ^ Coston-Clements, L., Settle, L.R., Hoss, D.E. and Cross, F.A. (1991) Utilization of the Sargassum habitat by marine invertebrates and vertebrates, a review. NOAA Technical Memorandum, volume 296, NMFS-SEFSC-296.
- ^ Stewart, Hannah Louise (2006). "Ontogenetic Changes in Buoyancy, Breaking Strength, Extensibility, and Reproductive Investment in a Drifting Macroalga Turbinaria Ornata (Phaeophyta)1". Journal of Phycology. 42: 43–50. doi:10.1111/j.1529-8817.2006.00184.x. S2CID 84580325.
- ^ a b Marshall, Harold G.; Burchardt, Lubomira (2005). "Neuston: Its definition with a historical review regarding its concept and community structure". Archiv für Hydrobiologie. 164 (4): 429–448. doi:10.1127/0003-9136/2005/0164-0429.
- ^ a b Franklin, Mark P.; McDonald, Ian R.; Bourne, David G.; Owens, Nicholas J. P.; Upstill-Goddard, Robert C.; Murrell, J. Colin (2005). "Bacterial diversity in the bacterioneuston (Sea surface microlayer): The bacterioneuston through the looking glass". Environmental Microbiology. 7 (5): 723–736. doi:10.1111/j.1462-2920.2004.00736.x. PMID 15819854.
- ^ a b Sieburth, John McN.; Willis, Paula-Jean; Johnson, Kenneth M.; Burney, Curtis M.; Lavoie, Dennis M.; Hinga, Kenneth R.; Caron, David A.; French, Frederick W.; Johnson, Paul W.; Davis, Paul G. (1976). "Dissolved Organic Matter and Heterotrophic Microneuston in the Surface Microlayers of the North Atlantic". Science. 194 (4272): 1415–1418. Bibcode:1976Sci...194.1415M. doi:10.1126/science.194.4272.1415. PMID 17819279. S2CID 24058391.
- ^ Taylor, Joe D.; Cunliffe, Michael (2014). "High-throughput sequencing reveals neustonic and planktonic microbial eukaryote diversity in coastal waters". Journal of Phycology. 50 (5): 960–965. doi:10.1111/jpy.12228. PMID 26988649. S2CID 1205582.
- ^ Upstill-Goddard, Robert C.; Frost, Thomas; Henry, Gordon R.; Franklin, Mark; Murrell, J. Colin; Owens, Nicholas J. P. (2003). "Bacterioneuston control of air-water methane exchange determined with a laboratory gas exchange tank". Global Biogeochemical Cycles. 17 (4): 1108. Bibcode:2003GBioC..17.1108U. doi:10.1029/2003GB002043. S2CID 97712481.
- ^ Tsyban, A. V. (1971). "Marine bacterioneuston". Journal of the Oceanographical Society of Japan. 27 (2): 56–66. doi:10.1007/BF02109331. S2CID 198202161.
- ^ Capone, Douglas G.; Zehr, Jonathan P.; Paerl, Hans W.; Bergman, Birgitta; Carpenter, Edward J. (1997). "Trichodesmium , a Globally Significant Marine Cyanobacterium". Science. 276 (5316): 1221–1229. doi:10.1126/science.276.5316.1221.
- ^ Ikawa, Terumi; Nozoe, Yuichi; Yamashita, Natsuko; Nishimura, Namiko; et al. (2018). "A Study of the Distributions of Two Endangered Sea Skaters Halobates matsumurai Esaki and Asclepios shiranui (Esaki) (Hemiptera: Gerridae: Halobatinae) with Special Reference to Their Strategies to Cope with Tidal Currents". Psyche: A Journal of Entomology. 2018. Hindawi Limited: 1–7. doi:10.1155/2018/3464829. ISSN 0033-2615.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Why are there so few insects at sea? Deutsche Welle, 9 July 2018.
- ^ a b c d Andersen, N. M.; Cheng, L. (2005). "The Marine Insect halobates (Heteroptera: Gerridae)". In Gibson, Robin (ed.). Oceanography and marine biology : an annual review. Boca Raton, Fla: CRC Press. ISBN 978-0-203-50781-0. OCLC 664909565.
- ^ Cheng, L (1985). "Biology of Halobates (Heteroptera: Gerridae)". Annual Review of Entomology. 30 (1). Annual Reviews: 111–135. doi:10.1146/annurev.en.30.010185.000551. ISSN 0066-4170. S2CID 86774669.
- ^ a b Herring, P. J. (1965). "Blue Pigment of a Surface-living Oceanic Copepod". Nature. 205 (4966): 103–104. Bibcode:1965Natur.205..103H. doi:10.1038/205103a0. S2CID 85081097.
- ^ a b c Zaitsev Y (1997). "Neuston of seas and oceans". In Liss PS (ed.). The sea surface and global change. Cambridge New York: Cambridge University Press. pp. 371–382. ISBN 978-0-521-56273-7. OCLC 34933503.
- ^ Ianora, A.; Santella, L. (1991). "Diapause embryos in the neustonic copepod Anomalocera patersoni". Marine Biology. 108 (3): 387–394. doi:10.1007/BF01313647. S2CID 85058107.
- ^ Jeong, Hyeon Gyeong; Suh, Hae-Lip; Yoon, Yang Ho; Choi, Im Ho; Soh, Ho Young (2008). "The first records of two neustonic calanoid copepods, pontella securifer and p. Sinica (Calanoida, pontellidae) in the south sea, korea". Ocean Science Journal. 43 (2): 91–100. Bibcode:2008OSJ....43...91J. doi:10.1007/BF03020585. S2CID 84647702.
- ^ a b c d e f Rahlff, Janina; Ribas-Ribas, Mariana; Brown, Scott M.; Mustaffa, Nur Ili Hamizah; et al. (31 July 2018). "Blue pigmentation of neustonic copepods benefits exploitation of a prey-rich niche at the air-sea boundary". Scientific Reports. 8 (1). Springer Science and Business Media LLC: 11510. Bibcode:2018NatSR...811510R. doi:10.1038/s41598-018-29869-7. ISSN 2045-2322. PMC 6068160. PMID 30065353.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ a b Svetlichny, Leonid; Larsen, Poul S.; Kiørboe, Thomas (2017). "Swim and fly. Escape strategy in neustonic and planktonic copepods". Journal of Experimental Biology. 221 (Pt 2). doi:10.1242/jeb.167262. PMID 29191859. S2CID 26677839.
- ^ a b Chae, J.; Nishida, S. (1994). "Integumental ultrastructure and color patterns in the iridescent copepods of the family Sapphirinidae (Copepoda: Poecilostomatoida)". Marine Biology. 119 (2): 205–210. doi:10.1007/BF00349558. S2CID 85268406.
- ^ Ianora, A.; Miralto, A.; Vanucci, S. (1992). "The surface attachment structure: A unique type of integumental formation in neustonic copepods". Marine Biology. 113 (3): 401–407. doi:10.1007/BF00349165. S2CID 84911783.
- ^ Engel, Anja; Bange, Hermann W.; Cunliffe, Michael; Burrows, Susannah M.; Friedrichs, Gernot; Galgani, Luisa; Herrmann, Hartmut; Hertkorn, Norbert; Johnson, Martin; Liss, Peter S.; Quinn, Patricia K.; Schartau, Markus; Soloviev, Alexander; Stolle, Christian; Upstill-Goddard, Robert C.; Van Pinxteren, Manuela; Zäncker, Birthe (2017). "The Ocean's Vital Skin: Toward an Integrated Understanding of the Sea Surface Microlayer". Frontiers in Marine Science. 4. doi:10.3389/fmars.2017.00165. hdl:10026.1/16046.
- ^ Sieburth, John McN.; Willis, Paula-Jean; Johnson, Kenneth M.; Burney, Curtis M.; Lavoie, Dennis M.; Hinga, Kenneth R.; Caron, David A.; French, Frederick W.; Johnson, Paul W.; Davis, Paul G. (1976). "Dissolved Organic Matter and Heterotrophic Microneuston in the Surface Microlayers of the North Atlantic". Science. 194 (4272): 1415–1418. Bibcode:1976Sci...194.1415M. doi:10.1126/science.194.4272.1415. PMID 17819279. S2CID 24058391.
- ^ Brodeur, Richard D. (1989). "Neustonic feeding by juvenile salmonids in coastal waters of the Northeast Pacific". Canadian Journal of Zoology. 67 (8): 1995–2007. doi:10.1139/z89-284.
- ^ Heinrich, A. K. (1971). "On the near-surface plankton of the eastern South Pacific Ocean". Marine Biology. 10 (4): 290–294. doi:10.1007/BF00368087. S2CID 85738413.
- ^ Heinrich, A. K. (2010). "Influence of the monsoon climate on the distribution of neuston copepods in the Northeastern Indian ocean". Oceanology. 50 (4): 549–555. Bibcode:2010Ocgy...50..549H. doi:10.1134/S0001437010040119. S2CID 128770397.
- ^ Turner, J.T., Collard, S.B., Wright, J.C., Mitchell, D.V. and Steele, P. (1979) "Summer distribution of pontellid copepods in the neuston of the eastern Gulf of Mexico continental shelf". Bulletin of Marine Science, 29(3): 287–297.
- ^ Maki, James S. (2003). "Neuston Microbiology: Life at the Air-Water Interface". Encyclopedia of Environmental Microbiology. doi:10.1002/0471263397.env234. ISBN 0471263397.
- ^ Humes, A. G. (1994) "How many copepods?" In: Ecology and Morphology of Copepods. Developments in Hydrobiology (Eds Ferrari F.D. and Bradley B.P.) Vol. 102 Springer, Dordrecht, 1–7.
- ^ Rahlff, Janina; Peters, Janna; Moyano, Marta; Pless, Ole; Claussen, Carsten; Peck, Myron A. (2017). "Short-term molecular and physiological responses to heat stress in neritic copepods Acartia tonsa and Eurytemora affinis". Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 203: 348–358. doi:10.1016/j.cbpa.2016.11.001. PMID 27825870.
- ^ Mauchline, J (1998). The biology of calanoid copepods. San Diego: Academic Press. ISBN 978-0-08-057956-6. OCLC 276935882.
- ^ a b Herring, P.J. (1967) "The pigments of plankton at the sea surface". In: Symp. Zool. Soc. Lond, 19: 215–235).
- ^ Zagalsky, P.F.; Herring, Peter J. (1972). "Studies on a carotenoprotein isolated from the copepod, Labidocera acutifrons and its relationship to the decapod carotenoproteins and other polyene-binding proteins". Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 41 (2): 397–415. doi:10.1016/0305-0491(72)90043-0.
- ^ Mojib, Nazia; Amad, Maan; Thimma, Manjula; Aldanondo, Naroa; Kumaran, Mande; Irigoien, Xabier (2014). "Carotenoid metabolic profiling and transcriptome‐genome mining reveal functional equivalence among blue‐pigmented copepods and appendicularia". Molecular Ecology. 23 (11): 2740–2756. doi:10.1111/mec.12781. hdl:10754/550807. PMID 24803335. S2CID 20245858.
- ^ Herring, P. J. (1965). "Blue Pigment of a Surface-living Oceanic Copepod". Nature. 205 (4966): 103–104. Bibcode:1965Natur.205..103H. doi:10.1038/205103a0. S2CID 85081097.
- ^ Caramujo, Maria-José; De Carvalho, Carla C. C. R.; Silva, Soraya J.; Carman, Kevin R. (2012). "Dietary Carotenoids Regulate Astaxanthin Content of Copepods and Modulate Their Susceptibility to UV Light and Copper Toxicity". Marine Drugs. 10 (12): 998–1018. doi:10.3390/md10050998. PMC 3397456. PMID 22822352.
- ^ Shagin, Dmitry A.; Barsova, Ekaterina V.; Yanushevich, Yurii G.; Fradkov, Arkady F.; Lukyanov, Konstantin A.; Labas, Yulii A.; Semenova, Tatiana N.; Ugalde, Juan A.; Meyers, Ann; Nunez, Jose M.; Widder, Edith A.; Lukyanov, Sergey A.; Matz, Mikhail V. (2004). "GFP-like Proteins as Ubiquitous Metazoan Superfamily: Evolution of Functional Features and Structural Complexity". Molecular Biology and Evolution. 21 (5): 841–850. doi:10.1093/molbev/msh079. PMID 14963095.
- ^ Abelló, Pere; Guerao, Guillermo; Codina, Meritxell (2004). "Distribution of the Neustonic Isopod Idotea Metallica in Relation to Shelf-Slope Frontal Structures". Journal of Crustacean Biology. 24 (4): 558–566. doi:10.1651/C-2505. S2CID 85806315.
- ^ Gutow, Lars; Franke, Heinz-Dieter (2001). "On the current and possible future status of the neustonic isopod Idotea metallica Bosc in the North Sea: A laboratory study" (PDF). Journal of Sea Research. 45 (1): 37–44. Bibcode:2001JSR....45...37G. doi:10.1016/S1385-1101(00)00058-7.
- ^ Hacker, S.D. and Madin, L.P. (1991) "Why habitat architecture and color are important to shrimps living in pelagic Sargassum: use of camouflage and plant-part mimicry". Marine ecology progress series, Oldendorf, 70(2): 143-155.
- ^ Hacker, SD; Madin, LP (1991). "Why habitat architecture and color are important to shrimps living in pelagic Sargassum: Use of camouflage and plant-part mimicry". Marine Ecology Progress Series. 70: 143–155. Bibcode:1991MEPS...70..143H. doi:10.3354/meps070143.
- ^ Miller, Michael (2009). "Ecology of Anguilliform Leptocephali: Remarkable Transparent Fish Larvae of the Ocean Surface Layer". Aqua-BioScience Monographs. 2 (4). doi:10.5047/absm.2009.00204.0001.
- ^ Brodeur, R.D., Pool, S.S. and Miller, T.W. (2013) "Prey selectivity of juvenile salmon on neustonic mesozooplankton in the northern California Current". North Pacific Anadromous Fish Commission, Technical Report, 9: 104-108.
- ^ Finn, Julian K.; Norman, Mark D. (2010). "The argonaut shell: Gas-mediated buoyancy control in a pelagic octopus". Proceedings of the Royal Society B: Biological Sciences. 277 (1696): 2967–2971. doi:10.1098/rspb.2010.0155. PMC 2982015. PMID 20484241.
- ^ Dall WH (1869) "Notes on the Argonaut". The American Naturalist, 3(5): 236–239
- ^ Macia, S. (2004). "New observations on airborne jet propulsion (Flight) in squid, with a review of previous reports". Journal of Molluscan Studies. 70 (3): 297–299. doi:10.1093/mollus/70.3.297.
- ^ a b c d e f g Wurl, Oliver; Ekau, Werner; Landing, William M.; Zappa, Christopher J. (1 January 2017). Deming, Jody W.; Bowman, Jeff (eds.). "Sea surface microlayer in a changing ocean – A perspective". Elementa: Science of the Anthropocene. 5. University of California Press. doi:10.1525/elementa.228. ISSN 2325-1026.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ a b c Carlson, David J. (1993). "The Early Diagenesis of Organic Matter: Reaction at the Air-Sea Interface". Organic Geochemistry. Topics in Geobiology. Vol. 11. pp. 255–268. doi:10.1007/978-1-4615-2890-6_12. ISBN 978-1-4613-6252-4.
- ^ a b Sieburth, John McN. (1983). "Microbiological and Organic-Chemical Processes in the Surface and Mixed Layers". Air-Sea Exchange of Gases and Particles. pp. 121–172. doi:10.1007/978-94-009-7169-1_3. ISBN 978-94-009-7171-4.
- ^ Zafiriou, Oliver C. (1986). "Photochemistry and the Sea-Surface Microlayer: Natural Processes and Potential as a Technique". Dynamic Processes in the Chemistry of the Upper Ocean. pp. 129–135. doi:10.1007/978-1-4684-5215-0_11. ISBN 978-1-4684-5217-4.
- ^ a b c Wurl, O.; Wurl, E.; Miller, L.; Johnson, K.; Vagle, S. (2011). "Formation and global distribution of sea-surface microlayers". Biogeosciences. 8 (1): 121–135. Bibcode:2011BGeo....8..121W. doi:10.5194/bg-8-121-2011.
- ^ Cunliffe, Michael; Engel, Anja; Frka, Sanja; Gašparović, Blaženka; Guitart, Carlos; Murrell, J Colin; Salter, Matthew; Stolle, Christian; Upstill-Goddard, Robert; Wurl, Oliver (2013). "Sea surface microlayers: A unified physicochemical and biological perspective of the air–ocean interface". Progress in Oceanography. 109: 104–116. Bibcode:2013PrOce.109..104C. doi:10.1016/j.pocean.2012.08.004.
- ^ Ellison, G. Barney; Tuck, Adrian F.; Vaida, Veronica (1999). "Atmospheric processing of organic aerosols". Journal of Geophysical Research: Atmospheres. 104 (D9): 11633–11641. Bibcode:1999JGR...10411633E. doi:10.1029/1999JD900073.
- ^ Liss, P. S. (1997). "Photochemistry of the sea-surface microlayer". The sea surface and global change. Cambridge New York: Cambridge University Press. pp. 383–424. ISBN 978-0-521-56273-7. OCLC 34933503.
- ^ Hunter, K. A. (1977) Chemistry of the sea-surface microlayer University of East Anglia. School of Environmental Sciences.
- ^ Zhang, Zhengbin (2003). "Direct determination of thickness of sea surface microlayer using a pH microelectrode at original location". Science in China Series B. 46 (4): 339. doi:10.1360/02yb0192.
- ^ Liss, P. S. (1997). "Chemistry of the sea-surface microlayer". The sea surface and global change. Cambridge New York: Cambridge University Press. ISBN 978-0-511-52502-5. OCLC 34933503.
- ^ Levich VG (1962) Physicochemical hydrodynamics, Prentice Hall International.
- ^ Schluessel, Peter; Emery, William J.; Grassl, Hartmut; Mammen, Theodor (1990). "On the bulk-skin temperature difference and its impact on satellite remote sensing of sea surface temperature". Journal of Geophysical Research. 95 (C8): 13341. Bibcode:1990JGR....9513341S. doi:10.1029/JC095iC08p13341. hdl:21.11116/0000-0004-BC37-B.
- ^ Carlson, David J. (1982). "A field evaluation of plate and screen microlayer sampling techniques". Marine Chemistry. 11 (3): 189–208. Bibcode:1982MarCh..11..189C. doi:10.1016/0304-4203(82)90015-9.
- ^ Cunliffe M, Wurl O.(2014) Guide to best practices to study the ocean's surface, Plymouth Occasional Publications of the Marine Biological Association of the United Kingdom.
- ^ Pattrick, Paula; Weidberg, Nicolas; Goschen, Wayne S.; Jackson, Jennifer M.; McQuaid, Christopher D.; Porri, Francesca (2021-05-31). "Larval Fish Assemblage Structure at Coastal Fronts and the Influence of Environmental Variability". Frontiers in Ecology and Evolution. 9. Frontiers Media SA. doi:10.3389/fevo.2021.684502. hdl:10037/23945. ISSN 2296-701X.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Apel, John R.; Byrne, H. Michael; Proni, John R.; Charnell, Robert L. (1975). "Observations of oceanic internal and surface waves from the earth resources technology satellite". Journal of Geophysical Research. 80 (6): 865–881. Bibcode:1975JGR....80..865A. doi:10.1029/JC080i006p00865.
- ^ Kingsford, M. J. (1990). "Linear oceanographic features: A focus for research on recruitment processes". Austral Ecology. 15 (4): 391–401. doi:10.1111/j.1442-9993.1990.tb01465.x.
- ^ Klymak, Jody; Legg, Sonya; Alford, Matthew; Buijsman, Maarten; Pinkel, Robert; Nash, Jonathan (2012). "The Direct Breaking of Internal Waves at Steep Topography". Oceanography. 25 (2): 150–159. doi:10.5670/oceanog.2012.50.
- ^ Engel, Anja; Bange, Hermann W.; Cunliffe, Michael; Burrows, Susannah M.; et al. (2017). "The Ocean's Vital Skin: Toward an Integrated Understanding of the Sea Surface Microlayer". Frontiers in Marine Science. 4. doi:10.3389/fmars.2017.00165. hdl:10026.1/16046. Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ a b Shanks, AL (1983). "Surface slicks associated with tidally forced internal waves may transport pelagic larvae of benthic invertebrates and fishes shoreward". Marine Ecology Progress Series. 13: 311–315. Bibcode:1983MEPS...13..311S. doi:10.3354/meps013311.
- ^ Jillett, J. B. & Zeldis, J. R. (1985) "Aerial observations of surface patchiness of a planktonic crustacean". Bull. Mar. Sci., 37: 609–619.
- ^ Kingsford, M. J.; Choat, J. H. (1986). "Influence of surface slicks on the distribution and onshore movements of small fish". Marine Biology. 91 (2): 161–171. doi:10.1007/BF00569432. S2CID 83769659.
- ^ l. Shanks, Alan; g. Wright, William (1987). "Internal-wave-mediated shoreward transport of cyprids, megalopae, and gammarids and correlated longshore differences in the settling rate of intertidal barnacles". Journal of Experimental Marine Biology and Ecology. 114: 1–13. doi:10.1016/0022-0981(87)90135-3.
- ^ Shanks, A. L. (1988) "Further support for the hypothesis that internal waves can cause shoreward transport of larval invertebrates and fish". Fish. Bull., 86: 703–714.
- ^ Kingsford, M. J.; Wolanski, E.; Choat, J. H. (1991). "Influence of tidally induced fronts and Langmuir circulations on distribution and movements of presettlement fishes around a coral reef". Marine Biology. 109: 167–180. doi:10.1007/BF01320244. S2CID 86057295.
- ^ Weidberg, N.; Lobón, C.; López, E.; García Flórez, L.; Fernández Rueda, MdP; Largier, J.; Acuña, JL (2014). "Effect of nearshore surface slicks on meroplankton distribution: Role of larval behaviour". Marine Ecology Progress Series. 506: 15–30. Bibcode:2014MEPS..506...15W. doi:10.3354/meps10777. hdl:10651/28404.
- ^ a b c d e f g Whitney, Jonathan L.; Gove, Jamison M.; McManus, Margaret A.; et al. (2021-02-04). "Surface slicks are pelagic nurseries for diverse ocean fauna". Scientific Reports. 11 (1). Springer Science and Business Media LLC: 3197. Bibcode:2021NatSR..11.3197W. doi:10.1038/s41598-021-81407-0. ISSN 2045-2322. PMC 7862242. PMID 33542255.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Leis JM, McCormick MI (2002). "The biology, behavior, and ecology of the pelagic, larval stage of coral reef fishes". In Sale P (ed.). Coral reef fishes: dynamics and diversity in a complex ecosystem. Amsterdam: Academic Press. pp. 171–199. ISBN 978-0-12-373609-3. OCLC 53963482.
- ^ Cowen RK (2002). "Oceanographic influences on larval dispersal and retention and their consequences for population connectivity". In Sale P (ed.). Coral reef fishes: dynamics and diversity in a complex ecosystem. Amsterdam: Academic Press. pp. 149–170. ISBN 978-0-12-373609-3. OCLC 53963482.
- ^ Doherty, Peter; Fowler, Tony (1994). "An Empirical Test of Recruitment Limitation in a Coral Reef Fish". Science. 263 (5149): 935–939. Bibcode:1994Sci...263..935D. doi:10.1126/science.263.5149.935. PMID 17758633. S2CID 30258297.
- ^ Armsworth, Paul R. (2002). "Recruitment Limitation, Population Regulation, and Larval Connectivity in Reef Fish Metapopulations". Ecology. 83 (4): 1092. doi:10.1890/0012-9658(2002)083[1092:RLPRAL]2.0.CO;2. ISSN 0012-9658.
- ^ Hobson, E.S. (1991) "Trophic relationships of fishes specialized to feed on zooplankters above coral reefs". In: The ecology of fishes on coral reefs, Academic Press, pages 69-95.
- ^ Boaden, A. E.; Kingsford, M.J (2015). "Predators drive community structure in coral reef fish assemblages". Ecosphere. 6 (4): 1–33. doi:10.1890/ES14-00292.1.
- ^ Gaffney, R. (2004) "Evaluation of the status of the recreational fishery for ulua in Hawaiʻi, and recommendations for future management". Hawaii Department of Land and Natural Resources, Division of Aquatic Resources Technical Report 20–02, 1–42.
- ^ Downie, RA; Babcock, RC; Thomson, DP; Vanderklift, MA (2013). "Density of herbivorous fish and intensity of herbivory are influenced by proximity to coral reefs". Marine Ecology Progress Series. 482: 217–225. Bibcode:2013MEPS..482..217D. doi:10.3354/meps10250.
- ^ Parrish, JD (1989). "Fish communities of interacting shallow-water habitats in tropical oceanic regions". Marine Ecology Progress Series. 58: 143–160. Bibcode:1989MEPS...58..143P. doi:10.3354/meps058143.
- ^ Brandl, Simon J.; Morais, Renato A.; Casey, Jordan M.; Parravicini, Valeriano; Tornabene, Luke; Goatley, Christopher H. R.; Côté, Isabelle M.; Baldwin, Carole C.; Schiettekatte, Nina M. D.; Bellwood, David R. (2019). "Response to Comment on "Demographic dynamics of the smallest marine vertebrates fuel coral reef ecosystem functioning"". Science. 366 (6472). doi:10.1126/science.aaz1301. PMID 31857447. S2CID 209424415.
- ^ Houde, E. D. (1997). "Patterns and trends in larval-stage growth and mortality of teleost fish". Journal of Fish Biology. 51: 52–83. doi:10.1111/j.1095-8649.1997.tb06093.x.
- ^ Haury, L. R.; McGowan, J. A.; Wiebe, P. H. (1978). "Patterns and Processes in the Time-Space Scales of Plankton Distributions". Spatial Pattern in Plankton Communities. pp. 277–327. doi:10.1007/978-1-4899-2195-6_12. ISBN 978-1-4899-2197-0.
- ^ Letcher, B. H.; Rice, J. A.; Crowder, L. B.; Rose, K. A. (1996). "Variability in survival of larval fish: Disentangling components with a generalized individual-based model". Canadian Journal of Fisheries and Aquatic Sciences. 53 (4): 787–801. doi:10.1139/f95-241.
- ^ Pineda, Jesús (1994). "Internal tidal bores in the nearshore: Warm-water fronts, seaward gravity currents and the onshore transport of neustonic larvae". Journal of Marine Research. 52 (3): 427–458. doi:10.1357/0022240943077046.
- ^ Shanks, Alan L.; Largier, John; Brink, Laura; Brubaker, John; Hooff, Rian (2000). "Demonstration of the onshore transport of larval invertebrates by the shoreward movement of an upwelling front". Limnology and Oceanography. 45 (1): 230–236. Bibcode:2000LimOc..45..230S. doi:10.4319/lo.2000.45.1.0230. S2CID 83672860.
- ^ Garland, Elizabeth D.; Zimmer, Cheryl Ann; Lentz, Steven J. (2002). "Larval distributions in inner‐shelf waters: The roles of wind‐driven cross‐shelf currents and diel vertical migrations". Limnology and Oceanography. 47 (3): 803–817. Bibcode:2002LimOc..47..803G. doi:10.4319/lo.2002.47.3.0803. S2CID 86452791.
- ^ Sponaugle, Su; Lee, Thomas; Kourafalou, Vassiliki; Pinkard, Deanna (2005). "Florida Current frontal eddies and the settlement of coral reef fishes". Limnology and Oceanography. 50 (4): 1033–1048. Bibcode:2005LimOc..50.1033S. doi:10.4319/lo.2005.50.4.1033. S2CID 16048164.
- ^ Greer, Adam T.; Cowen, Robert K.; Guigand, Cedric M.; Hare, Jonathan A.; Tang, Dorothy (2014). "The role of internal waves in larval fish interactions with potential predators and prey". Progress in Oceanography. 127: 47–61. Bibcode:2014PrOce.127...47G. doi:10.1016/j.pocean.2014.05.010.
- ^ Shulzitski, Kathryn; Sponaugle, Su; Hauff, Martha; Walter, Kristen; d'Alessandro, Evan K.; Cowen, Robert K. (2015). "Close encounters with eddies: Oceanographic features increase growth of larval reef fishes during their journey to the reef". Biology Letters. 11 (1). doi:10.1098/rsbl.2014.0746. PMC 4321146. PMID 25631227.
- ^ Shulzitski, Kathryn; Sponaugle, Su; Hauff, Martha; Walter, Kristen D.; Cowen, Robert K. (2016). "Encounter with mesoscale eddies enhances survival to settlement in larval coral reef fishes". Proceedings of the National Academy of Sciences. 113 (25): 6928–6933. Bibcode:2016PNAS..113.6928S. doi:10.1073/pnas.1601606113. PMC 4922168. PMID 27274058.
- ^ Woodson, C. Brock; Litvin, Steven Y. (2015). "Ocean fronts drive marine fishery production and biogeochemical cycling". Proceedings of the National Academy of Sciences. 112 (6): 1710–1715. Bibcode:2015PNAS..112.1710W. doi:10.1073/pnas.1417143112. PMC 4330775. PMID 25624488.
- ^ a b Larson, Ronald J. (1980). "The Medusa of Velella velella (Linnaeus, 1758) (Hydrozoa, Chondrophorae)". Journal of Plankton Research. 2 (3): 183–186. doi:10.1093/plankt/2.3.183.
- ^ Štorkánová, Hana; Oreská, Sabína; Špiritović, Maja; Heřmánková, Barbora; Bubová, Kristýna; Komarc, Martin; Pavelka, Karel; Vencovský, Jiří; Distler, Jörg H. W.; Šenolt, Ladislav; Bečvář, Radim; Tomčík, Michal (2021). "Plasma Hsp90 levels in patients with systemic sclerosis and relation to lung and skin involvement: A cross-sectional and longitudinal study". Scientific Reports. 11 (1): 1. Bibcode:2021NatSR..11....1S. doi:10.1038/s41598-020-79139-8. PMC 7791137. PMID 33414495.
- ^ a b Brinckmann-Voss, A. (1970) Anthomedusae, Athecatae:(Hydrozoa, Cnidaria) of the Mediterranean. 1. Capitata. Stazione zoologica.
- ^ Laursen, D. (1953) The genus Ianthina: a monograph. The Carlsberg Foundation's Oceanographical Expedition Round the World 1928–30 and Previous "Dana" Expeditions. CA Reitzels.
- ^ Miller Andersen N, Cheng L (2010) "The Marine Insecthalobates(Heteroptera: Gerridae)". In: Oceanography and Marine Biology. CRC Press, 119–179.
- ^ Leloup E. (1929) "Research on the anatomy and development of Velella spirans Forsk". Liege.
- ^ Delsman, H.C. (1923) "Beiträge zur Entwickelungsgeschichte von Porpita (Contributions to the history of the development of Porpita)". Treubia, 3: 243-266.
- ^ Wilson, Douglas P.; Wilson, M. Alison (1956). "A contribution to the biology of Ianthina janthina (L.)" (PDF). Journal of the Marine Biological Association of the United Kingdom. 35 (2): 291–305. doi:10.1017/S0025315400010146. S2CID 83752461.
- ^ Lalli, Carol (1989). Pelagic snails : the biology of holoplanktonic gastropod mollusks. Stanford, Calif: Stanford University Press. ISBN 978-0-8047-1490-7. OCLC 18256759.
- ^ Gower, J. F. R.; King, S. A. (2011). "Distribution of floating Sargassum in the Gulf of Mexico and the Atlantic Ocean mapped using MERIS". International Journal of Remote Sensing. 32 (7): 1917–1929. Bibcode:2011IJRS...32.1917G. doi:10.1080/01431161003639660. S2CID 130180590.
- ^ Woltereck R. (1904) "Ueber die Entwicklung der Velella aus einer in der tiefe vorkommenden Larve". Fischer.
- ^ Savilov, A.I. (1969) "Pleuston of the Pacific ocean". Biology of the Pacific Ocean, 264-353.
- ^ Bieri, R. (19770 "The ecological significance of seasonal occurrence and growth rate of Velella (Hydrozoa)". Publications of the Seto Marine Biological Laboratory, 24(1–3): 63-76.
- ^ Savilov, A.I. (1969) "Pleuston of the Pacific ocean". In: Biology of the Pacific Ocean, pages 264-353.
- ^ Living Bacteria Are Riding Earth’s Air Currents Smithsonian Magazine, 11 January 2016.
- ^ Robbins, Jim (13 April 2018). "Trillions Upon Trillions of Viruses Fall From the Sky Each Day". The New York Times. Retrieved 14 April 2018.
- ^ Reche, Isabel; D’Orta, Gaetano; Mladenov, Natalie; Winget, Danielle M; Suttle, Curtis A (29 January 2018). "Deposition rates of viruses and bacteria above the atmospheric boundary layer". ISME Journal. 12 (4): 1154–1162. doi:10.1038/s41396-017-0042-4. PMC 5864199. PMID 29379178.
- ^ A. C. Hardy and P. S. Milne (1938) Studies in the Distribution of Insects by Aerial Currents. Journal of Animal Ecology, 7(2):199-229
- ^ Després, Vivianer.; Huffman, J.Alex; Burrows, Susannah M.; Hoose, Corinna; Safatov, Aleksandrs.; Buryak, Galina; Fröhlich-Nowoisky, Janine; Elbert, Wolfgang; Andreae, Meinrato.; Pöschl, Ulrich; Jaenicke, Ruprecht (2012). "Primary biological aerosol particles in the atmosphere: A review". Tellus B: Chemical and Physical Meteorology. 64: 15598. Bibcode:2012TellB..6415598D. doi:10.3402/tellusb.v64i0.15598. S2CID 98741728.
- ^ Wiśniewska, K.; Lewandowska, A.U.; Śliwińska-Wilczewska, S. (2019). "The importance of cyanobacteria and microalgae present in aerosols to human health and the environment – Review study". Environment International. 131: 104964. doi:10.1016/j.envint.2019.104964. PMID 31351382.
- ^ a b Moustaka-Gouni, Maria; Kormas, K. A.; Moustaka-Gouni, M. (2011). "Airborne Algae and Cyanobacteria Occurrence and Related Health Effects". Frontiers in Bioscience. 3 (2): 772–787. doi:10.2741/e285. PMID 21196350.
- ^ Bernstein, I.Leonard; Safferman, Robert S. (1966). "Sensitivity of skin and bronchial mucosa to green algae". Journal of Allergy. 38 (3): 166–173. doi:10.1016/0021-8707(66)90039-6. PMID 5223702.
- ^ Hoose, C.; Möhler, O. (2012). "Heterogeneous ice nucleation on atmospheric aerosols: A review of results from laboratory experiments". Atmospheric Chemistry and Physics. 12 (20): 9817–9854. Bibcode:2012ACP....12.9817H. doi:10.5194/acp-12-9817-2012.
- ^ Tesson, Sylvie V. M.; Šantl-Temkiv, Tina (2018). "Ice Nucleation Activity and Aeolian Dispersal Success in Airborne and Aquatic Microalgae". Frontiers in Microbiology. 9: 2681. doi:10.3389/fmicb.2018.02681. PMC 6240693. PMID 30483227.
- ^ Sharma, Naveen Kumar; Rai, Ashwani K. (2008). "Allergenicity of airborne cyanobacteria Phormidium fragile and Nostoc muscorum". Ecotoxicology and Environmental Safety. 69 (1): 158–162. doi:10.1016/j.ecoenv.2006.08.006. PMID 17011621.
- ^ Lewandowska, Anita Urszula; Śliwińska-Wilczewska, Sylwia; Woźniczka, Dominika (2017). "Identification of cyanobacteria and microalgae in aerosols of various sizes in the air over the Southern Baltic Sea". Marine Pollution Bulletin. 125 (1–2): 30–38. Bibcode:2017MarPB.125...30L. doi:10.1016/j.marpolbul.2017.07.064. PMID 28823424.
- ^ Dommergue, Aurelien; Amato, Pierre; Tignat-Perrier, Romie; Magand, Olivier; Thollot, Alban; Joly, Muriel; Bouvier, Laetitia; Sellegri, Karine; Vogel, Timothy; Sonke, Jeroen E.; Jaffrezo, Jean-Luc; Andrade, Marcos; Moreno, Isabel; Labuschagne, Casper; Martin, Lynwill; Zhang, Qianggong; Larose, Catherine (2019). "Methods to Investigate the Global Atmospheric Microbiome". Frontiers in Microbiology. 10: 243. doi:10.3389/fmicb.2019.00243. PMC 6394204. PMID 30967843.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.